Their analysis provides essential information for vaccine design and the evaluation of diagnostics and vaccine candidates

Credit: Grifoni et al./Cell Host & Microbe
LA JOLLA, CA–Within two months, SARS-CoV-2, a previously unknown coronavirus, has raced around globe, infecting over a 100,000 people with numbers continuing to rise quickly. Effective countermeasures require helpful tools to monitor viral spread and understand how the immune system responds to the virus.
Publishing in the March 16, 2020, online issue of Cell, Host and Microbe, a team of researchers at La Jolla Institute for Immunology, in collaboration with researchers at the J. Craig Venter Institute, provides the first analysis of potential targets for effective immune responses against the novel coronavirus. The researchers used existing data from known coronaviruses to predict which parts of SARS-CoV-2 are capable of activating the human immune system.
When the immune system encounters a bacterium or a virus, it zeroes in on tiny molecular features, so called epitopes, which allow cells of the immune system to distinguish between closely related foreign invaders and focus their attack. Having a complete map of viral epitopes and their immunogenicity is critical to researchers attempting to design new or improved vaccines to protect against COVID-19, the disease caused by SARS-CoV-2.
“Right now, we have limited information about which pieces of the virus elicit a solid human response,” says the study’s lead author Alessandro Sette, Dr. Biol.Sci, a professor in the Center for Infectious Disease and Vaccine Research at LJI. “Knowing the immunogenicity of certain viral regions, or in other words, which parts of the virus the immune system reacts to and how strongly, is of immediate relevance for the design of promising vaccine candidates and their evaluation.”
While scientists currently know very little about how the human immune system responds to SARS-CoV-2, the immune response to other coronaviruses has been studied and a significant amount of epitope data is available.
Four other coronaviruses are currently circulating in the human population. They cause generally mild symptoms and together they are responsible for an estimated one quarter of all seasonal colds. But every few years, a new coronavirus emerges that causes severe disease as was the case with SARS-CoV in 2003 and MERS-CoV in 2008, and now SARS-CoV-2.
“SARS-CoV-2 is most closely related to SARS-CoV, which also happens to be the best characterized coronavirus in terms of epitopes,” explains first author Alba Grifoni, Ph.D, a postdoctoral researcher in the Sette lab.
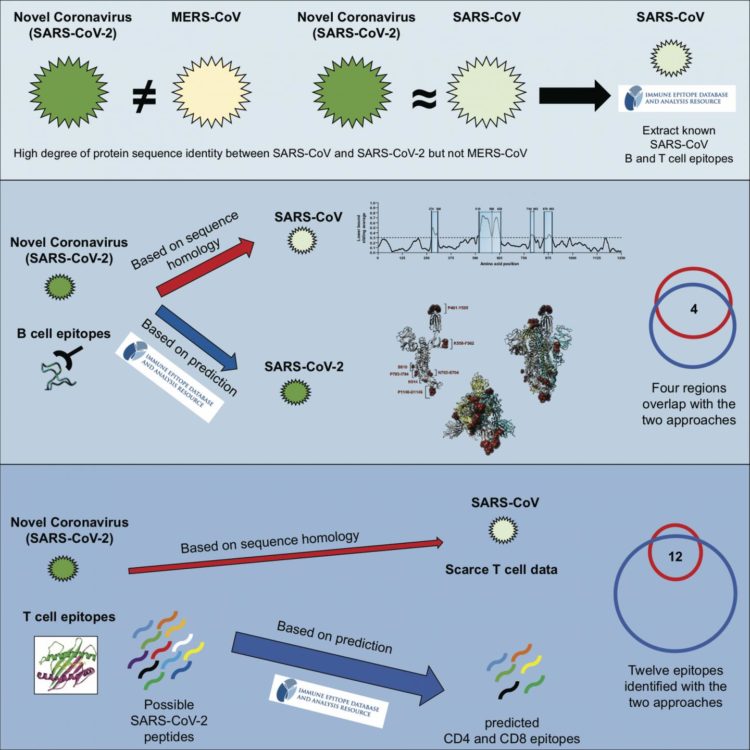
For their study, the authors used available data from the LJI-based Immune Epitope Database (IEDB), which contains over 600,000 known epitopes from some 3,600 different species, and the Virus Pathogen Resource (ViPR), a complementary repository of information about pathogenic viruses. The team compiled known epitopes from SARS-CoV and mapped the corresponding regions to SARS-CoV-2.
“We were able to map back 10 B cell epitopes to the new coronavirus and because of the overall high sequence similarity between SARS-CoV and SARS-CoV-2, there is a high likelihood that the same regions that are immunodominant in SARS-CoV are also dominant in SARS-CoV-2 is,” says Grifoni.
Five of these regions were found in the spike glycoprotein, which forms the “crown” on the surface of the virus that gave coronaviruses their name; two in the membrane protein, which is embedded in the membrane that envelopes the protective protein shell around the viral genome and three in the nucleoprotein, which forms the shell.
In a similar analysis, T cell epitopes were also mostly associated with the spike glycoprotein and nucleoprotein.
In a completely different approach, Grifoni used the epitope prediction algorithm hosted by the IEDB to predict linear B cell epitopes. A recent study by scientists at the University of Texas Austin determined the three-dimensional structure of the spike proteins, which allowed the LJI team to take the protein’s spatial architecture into account when predicting epitopes. This approach confirmed two of the likely epitope regions they had predicted earlier.
To substantiate the SARS-CoV-2 T cell epitopes identified based on their homology to SARS-CoV, Grifoni compared them with epitopes pinpointed by the Tepitool resource in the IEDB. Using this approach, she was able verify 12 out of 17 SARS-CoV-2 T cell epitopes identified based on sequence similarities to SARS-CoV.
“The fact that we found that many B and T cell epitopes are highly conserved between SARS-CoV and SARS-CoV-2 provides a great starting point for vaccine development,” says Sette. “Vaccine strategies that specifically target these regions could generate immunity that’s not only cross-protective but also relatively resistant to ongoing virus evolution.”
###
The work was funded in part by the National Institute of Allergy and Infectious Diseases, a component of the National Institutes of Health through contracts 75N9301900065, 75N93019C00001 and 75N93019C00076.
Full citation:
Alba Grifoni, John Sidney, Yun Zhang, Richard H Scheuermann, Bjoern Peters and Alessandro Sette. A Sequence Homology and Bioinformatic Approach Can Predict Candidate Targets for Immune Responses to SARS-CoV-2. Cell, Host and Microbe, 2020.
https:/
A pre-proof is available here.
About La Jolla Institute for Immunology
The La Jolla Institute for Immunology is dedicated to understanding the intricacies and power of the immune system so that we may apply that knowledge to promote human health and prevent a wide range of diseases. Since its founding in 1988 as an independent, nonprofit research organization, the Institute has made numerous advances leading toward its goal: life without disease.
Link to University of Texas study :
https:/
Media Contact
[email protected]
[email protected]
858-357-7481
Related Journal Article
http://dx.