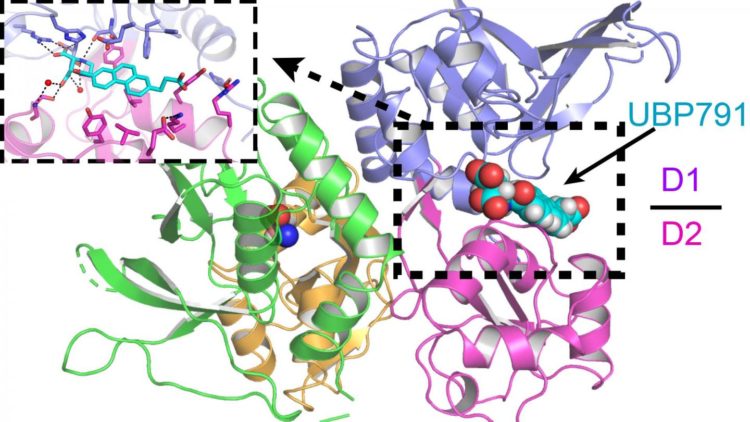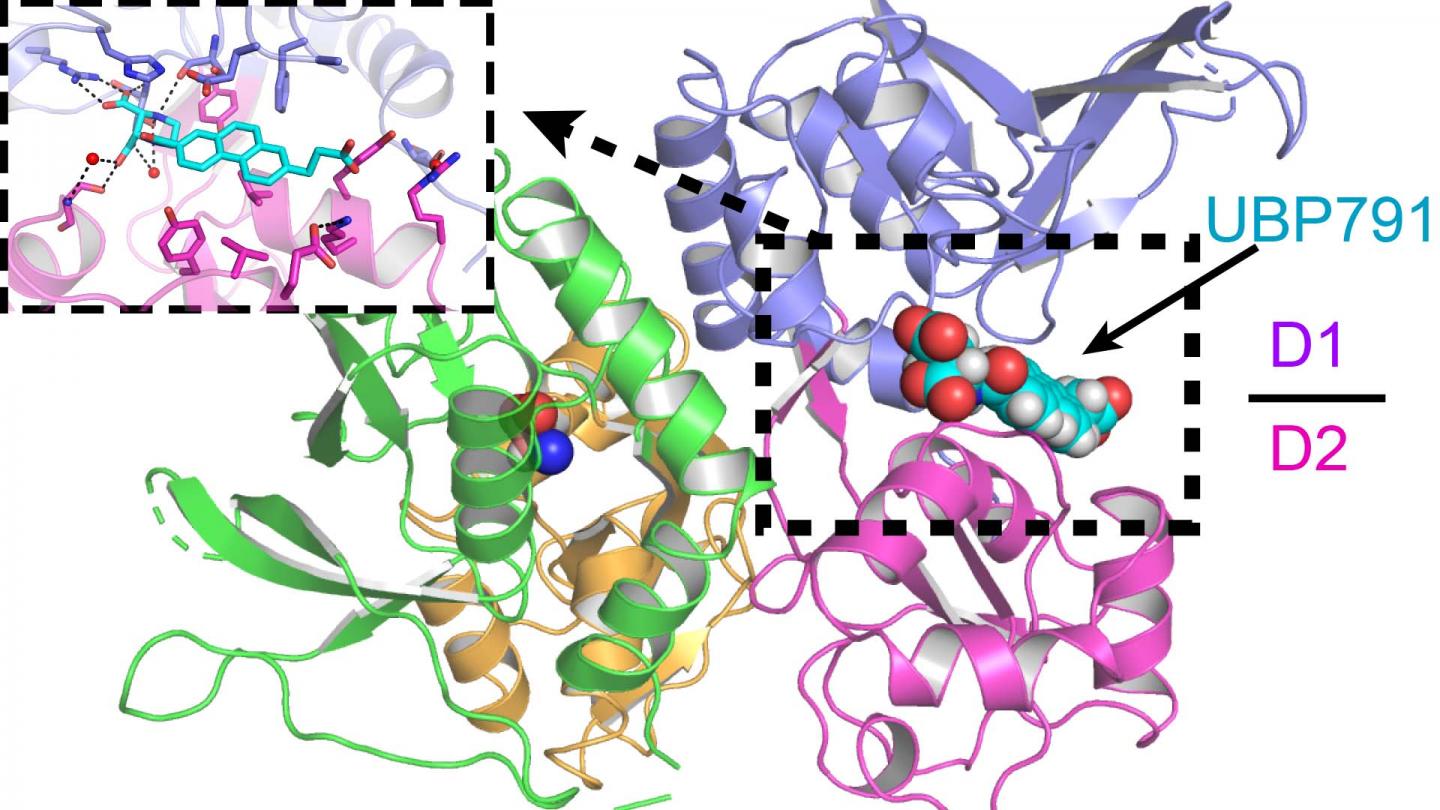
Credit: Furukawa lab/CSHL, 2020
NMDARs (N-methyl-D-aspartate receptors) serve as valves on nerve cells, controlling the flow of electrical signals in the brain. This special group of receptors is suspect in many neurological diseases, including Alzheimer’s, epilepsy, stroke, and Parkinson’s. Biologists from Cold Spring Harbor Laboratory (CSHL) and chemists from the University of Bristol have joined forces, creating a chemical compound to enable more precise investigation of NMDAR activity.
In the latest issue of Nature Communications, CSHL Professor Hiro Furukawa and colleagues detailed how they identified and perfected a chemical compound that inhibits, or stops the activity of certain NMDARs. By inhibiting some NMDARs while letting others function, researchers can now identify the roles different types of NMDA receptors play in both healthy and diseased brains.
Jue Xiang Wang, a graduate of CSHL’s Ph.D. program who helped lead the research, explained that the CSHL-Bristol team investigated how the novel compound UBP791 targets a pair of NMDAR subunits called GluN2C and GluN2D.
“There is evidence that GluN2C and GluN2D are relevant in the same brain regions where motor functions are affected by Parkinson’s disease,” she said. “Without good inhibitors, we could only speculate on what the 2C and 2D receptors do.”
By inhibiting the activity of GluN2C and GluN2D receptors with higher efficiency and specificity than before, scientists can better study the role that they play in Parkinson’s.
Video: https:/
Furukawa’s lab worked with Professor David Jane’s chemistry lab at the University of Bristol to improve the NMDAR-targeting compound. The CSHL lab specializes in visualizing the physical structure of NMDARs using a technique called X-ray crystallography. Knowing the structure of the receptor was critical for the chemists, who were then able to design UBP791 to connect specifically with the GluN2C and GluN2D receptors much like how a key is made to fit into specific locks. Studying what makes UBP791 fit particularly well further allowed the scientists to improve the compound, creating its latest version, UBP1700.
The UBP1700 compound is more precise than any of its predecessors and “it’s also more potent,” said Wang. “That’s important because researchers will only need small amounts of the compound to shut down the targeted receptors. This limits the potential for side-effects that the compound might produce.”
Moving forward, Furukawa’s lab and their Bristol collaborators will be working on further refining the new compound for use in research.
###
Media Contact
Sara Roncero-Menendez
[email protected]
516-367-6866
Related Journal Article
http://dx.