Penn study finds blocking the ATF4 gene causes tumors to die from too much stress
Credit: Penn Medicine
PHILADELPHIA – For years, researchers have been trying to target a gene called MYC that is known to drive tumor growth in multiple cancer types when it is mutated or over-expressed, but hitting that target successfully has proven difficult. Now researchers in the Perelman School of Medicine of the University of Pennsylvania have identified a new pathway that works as a partner to MYC and may be its Achilles’ Heel. The pathway involves a protein called ATF4, and when it’s blocked, it can cause cancer cells to produce too much protein and die. These findings in cell lines and mouse models could point the way toward a new therapeutic approach as inhibitors that can block synthesis of ATF4 already exist. The journal Nature Cell Biology published the findings today.
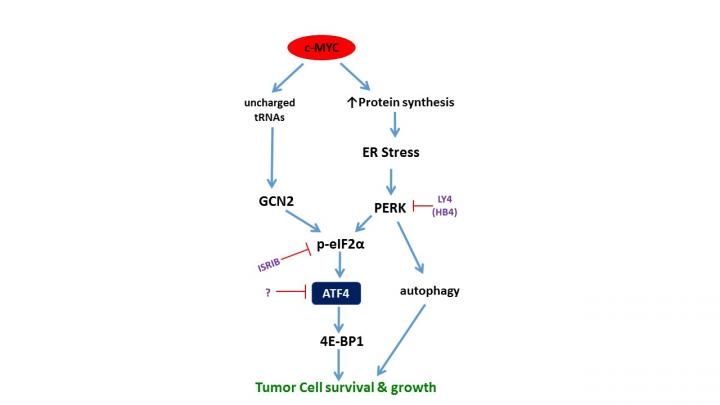
MYC is a gene that controls normal cell growth, but when it is mutated or amplified in cancer, it sets off a chain reaction that helps tumors grow uncontrollably. While there is currently no specific way to target it, previous research has focused on blocking other steps in the chain as a workaround to impede tumor growth. The team, led by Constantinos Koumenis, PhD, the Richard Chamberlain Professor of Radiation Oncology and vice chair and research division director of Radiation Oncology, have previously shown that in certain tumors, one of these steps is regulated by a kinase called PERK, which activates ATF4. However, in this new study, they’ve shown that blocking PERK does not always stop tumor growth because MYC actually controls a second process that can work in parallel as a redundancy in the system. This study identified this second kinase, which is called GCN2.
“What we’ve learned is that we need to go further downstream to block tumor growth in a way that cancer cells can’t easily escape, and our study identifies the target to do just that,” said Koumenis, who is the co-senior author on this study along with Davide Ruggero, PhD, a professor of Urology in the Helen Diller Family Comprehensive Cancer Center at the University of California, San Francisco (UCSF).
This study shows the alternative approach is to target ATF4 itself, since it’s the point where both signal pathways converge, meaning there’s less redundancy built in to allow cancer to survive. The findings also show that ATF4 turns on the genes MYC needs for growth and also controls the rate at which cells make specific proteins called 4E-BP. When the researchers knocked out ATF4 in cells or mice, they found tumor cells continued to build up those proteins and eventually died as a result of stress. This blocked tumor growth in mice with lymphomas and colorectal cancer. This study also found that when tumors in humans are driven by MYC, ATF4 and its protein partner 4E-BP are also overly expressed, which is further evidence that these findings may point to an approach that could work for humans.
“This shows us the potential impacts of targeting ATF4 in MYC-dependent tumors, something we’re already studying. We’re also working to confirm this approach will not cause any serious off-target effects,” said lead author Feven Tameire, PhD, who conducted this research while she was a doctoral candidate at Penn.
Researchers say future studies will also focus on continuing to investigate why ATF4 works the way it does, which may help their understanding of whether there are other potential targets in the chain.
###
Other Penn authors on this study include Ioannis I. Verginadis, Nektaria Maria Leli, Rani Ojha, Carlo Salas, Frank Chinga, Alexandra. M. Monroy, Weixuan Fu, Paul Wang, Ravi K. Amaravadi, and Serge Y. Fuchs. Authors from other institutions include J. Alan Diehl. From the Hollings Cancer Center at the University of South Carolina, Christine Polte and Zoya Ignatova from the University of Hamburg, Crystal S. Conn from UCSF, Andrew Kossenkov from The Wistar Institute, and Jiangbin Ye from Stanford University.
This study was supported by the National Institutes of Health (P01CA165997, 5R01CA198015-04, F31CA183569).
Penn Medicine is one of the world’s leading academic medical centers, dedicated to the related missions of medical education, biomedical research, and excellence in patient care. Penn Medicine consists of the Raymond and Ruth Perelman School of Medicine at the University of Pennsylvania (founded in 1765 as the nation’s first medical school) and the University of Pennsylvania Health System, which together form a $7.8 billion enterprise.
The Perelman School of Medicine has been ranked among the top medical schools in the United States for more than 20 years, according to U.S. News & World Report’s survey of research-oriented medical schools. The School is consistently among the nation’s top recipients of funding from the National Institutes of Health, with $425 million awarded in the 2018 fiscal year.
The University of Pennsylvania Health System’s patient care facilities include: the Hospital of the University of Pennsylvania and Penn Presbyterian Medical Center–which are recognized as one of the nation’s top “Honor Roll” hospitals by U.S. News & World Report–Chester County Hospital; Lancaster General Health; Penn Medicine Princeton Health; and Pennsylvania Hospital, the nation’s first hospital, founded in 1751. Additional facilities and enterprises include Good Shepherd Penn Partners, Penn Home Care and Hospice Services, Lancaster Behavioral Health Hospital, and Princeton House Behavioral Health, among others.
Penn Medicine is powered by a talented and dedicated workforce of more than 40,000 people. The organization also has alliances with top community health systems across both Southeastern Pennsylvania and Southern New Jersey, creating more options for patients no matter where they live.
Penn Medicine is committed to improving lives and health through a variety of community-based programs and activities. In fiscal year 2018, Penn Medicine provided more than $525 million to benefit our community.
Media Contact
John Infanti
[email protected]
Related Journal Article
http://dx.




