Credit: Image created with contributions from Clemens Kratochwil, Paul Flechsig, Thomas Lindner, Labidi Abderrahim, Annette Altmann, Walter Mier, Sebastian Adeberg, Hendrik Rathke, Manuel Röhrich, Hauke Winter, Peter Plinkert, Frederik Marme, Matthias…
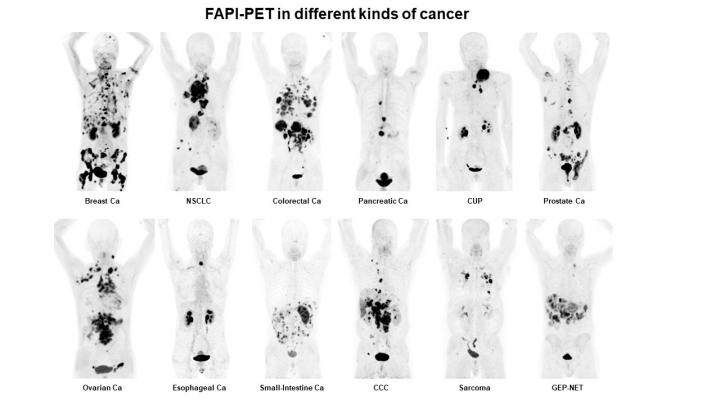
A single radiotracer can identify nearly 30 types of cancer, allowing for new applications in noninvasive diagnosis, staging and treatment, according to research presented at the 2019 Annual Meeting of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). The results of the study demonstrate that positron emission tomography/computed tomography (PET/CT) with a fibroblast-activation protein inhibitor (FAPI)–which targets the overexpressed proteins present in cancer–resulted in images with exceptionally clear tumor delineation and high image contrast, as demonstrated in the 68Ga-FAPI-PET/CT image that has been selected as the 2019 SNMMI Image of the Year.
Each year, the SNMMI chooses an image that best exemplifies the most promising advances in the field of nuclear medicine and molecular imaging. The state-of-the-art technologies captured in these images demonstrate the capacity to improve patient care by detecting disease, aiding diagnosis, improving clinical confidence and providing a means of selecting appropriate treatments. This year, the SNMMI Henry N. Wagner, Jr., Image of the Year was chosen from more than 2,300 abstracts submitted to the meeting and voted on by reviewers and the society leadership.
This honor goes to a team of researchers at University Hospital Heidelberg, Germany. Showcasing the efficacy of the FAPI radiotracer, the image demonstrates the uptake of the FAPI in 12 epidemiological tumor entities, including high uptake values in lung, breast, prostate, esophageal and pancreatic cancer. Low background activity was also observed in the various cancers, resulting in high image contrast and excellent tumor delineation. Also, in contrast to FDG-PET/CT, FAPI-PET/CT can be performed without specific patient preparation (fasting, recline during uptake time) after a very short uptake time (~10 min.) and might improve patient comfort and accelerate workflow.
In addition to identifying tumors, the research is also important for the development of cancer treatments in the future. “Immunotherapies can be highly effective in some patients and without any anti-tumor activity in other patients,” noted author Uwe Haberkorn, MD, professor and chair of nuclear medicine at the University Hospital of Heidelberg and the German Cancer Research Center in Heidelberg, Germany. “Currently, predictive biomarkers for appropriate patient selection are limited. Due to its biological role, FAP-targeted diagnostics has the potential to be a predictive biomarker.”
Prof. Haberkorn continued by noting that “The used FAP-ligands contain the DOTA-chelator, which enables labeling with therapeutic radionuclides. The observation that the ligand accumulates in several important tumor-entities potentially indicates a huge field of therapeutic application to be evaluated in the future.”
Furthermore, FAPI-PET already has the potential to add important diagnostic value, particularly in challenging FDG-PET cancer subtypes such as pancreas, ovarian and colorectal cancer and also in patients with peritoneal cancer spread. FAPI-PET might even promote a new route in non-cancer diseased patients such as chronic inflammatory pathogenesis or myocardial infarction, according to Frederik L. Giesel, MD, vice chair of nuclear medicine at the University Hospital Heidelberg.
Umar Mahmood, MD, PhD, chair of the SNMMI Scientific Program Committee, noted, “Importantly and positively for patients suffering with cancer, the last decade has seen a marked acceleration in our understanding of the drivers of cancer. This has led to the development of therapies that specifically target distinct cells in the tumor microenvironment. The image of the year highlights the novel ability to image a druggable protein expressed by reactive fibroblasts found in a variety of tumors.”
“Imaging with new tracers such as the one developed–and very nicely demonstrated in this study in a wide variety of cancer types–shows the power of molecular imaging to characterize tumors,” Mahmood continued. “The image of the year epitomizes the great progress made in our field in developing new imaging agents to help optimize cancer therapy for individual patients through such non-invasive characterization.”
###
Abstract 289. “Intensity of tracer-uptake in FAPI-PET/CT in different kinds of cancer,” Frederik Giesel, Paul Flechsig, Labidi Abderrahim, Manuel Röhrich, Annette Altmann, Anastasia Loktev and Thomas Lindner, Department of Nuclear Medicine, University Hospital Heidelberg, Heidelberg, Germany; Hendrik Rathke, Uwe Haberkorn and Clemens Kratochwil, University Hospital Heidelberg, Heidelberg, Germany; Juergen Debus, Radio-Oncology, University Hospital Heidelberg, Heidelberg, Germany; and Dirk Jaeger, NCT, Heidelberg, Germany.
66TH SNMMI Annual Meeting, June 22-25, 2019, Anaheim, CA.
To schedule an interview with the researchers, please contact David Harrison at (410) 804-1728 or [email protected]. All 2019 SNMMI Annual Meeting abstracts can be found online at http://jnm.
About the Society of Nuclear Medicine and Molecular Imaging
The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging, vital elements of precision medicine that allow diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes.
SNMMI’s more than 17,000 members set the standard for molecular imaging and nuclear medicine practice by creating guidelines, sharing information through journals and meetings and leading advocacy on key issues that affect molecular imaging and therapy research and practice. For more information, visit http://www.
Media Contact
David Harrison
[email protected]




