PET imaging effective in detecting tau deposits in progressive supranuclear palsy
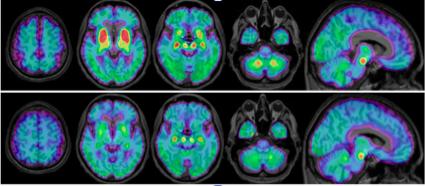
Credit: Brendel M, Barthel H, Van Eimeren T, et al.
ANAHEIM, CA (Embargoed until 4:30 p.m. PDT, Saturday, June 22, 2019) – Researchers have discovered a novel radioligand that can effectively differentiate progressive supranuclear palsy (PSP) from similar brain disorders, allowing for earlier and more reliable diagnosis of the disease. Presented at the Society of Nuclear Medicine and Molecular Imaging’s 2019 Annual Meeting, these findings bring physicians a step closer to being able to definitively diagnose PSP with imaging rather than waiting for confirmation upon autopsy.
PSP is a rare brain disorder characterized by loss of balance, blurred vision and uncontrolled eye movement, slurred speech, cognitive decline and mood changes. The disease worsens over time, and there is no cure. Often, PSP symptoms are similar to Parkinson’s disease and dementia, making it hard to diagnose.
“Currently, PSP can only be definitively diagnosed post-mortem by examining region-specific tau deposits in the brain,” said Matthias Brendel, MD, MHBA, at Ludwig-Maximilians-University of Munich in Germany. “Future interventional trials targeting tau in PSP would strongly benefit from biomarkers to validate the specific presence of the tau deposits and to monitor treatment response during therapy.”
In the study, researchers utilized a novel second-generation radioligand, 18F-PI-2620, to evaluate patients with suspected tau pathology in clinically diagnosed PSP. Seventeen patients with probable or possible PSP underwent 18F-PI-2620 positron emission tomography (PET) imaging at four different health care centers, along with 10 healthy control patients and seven disease control patients who had multi-system atrophy, Parkinson’s disease or Alzheimer’s disease. Standardized uptake value (SUV) ratios of 18F-PI-2620 in predetermined brain areas were obtained and compared between the PSP, healthy control and disease control patients. In addition, disease severity, measured by the PSP rating scale, was correlated with PET findings.
A significantly elevated mean 18F-PI-2620 SUV ratio was found in the globus pallidus and the substantia nigra areas of the brain in the PSP patients as compared to the healthy control group. In contrast, the disease control group showed similar or only slightly elevated SUV ratios when compared to the healthy control group. Furthermore, results showed that even patients with low disease severity already had significantly elevated 18F-PI-2620 uptake in the globus pallidus when compared to the healthy control group.
“My colleagues and I were able to detect an elevated signal in the majority of evaluated PSP patients and could clearly discriminate the PSP group from healthy controls and disease controls,” noted Brendel. “Importantly, PSP patients at early disease stages also revealed an elevated PI-2620 signal, which points at the suitability of this ligand as an early PSP biomarker. Detection of tau in PSP patients by PI-2620 could play a role in future anti-tau trials for this disease, and molecular imaging could serve to select the right patients for targeted therapies.”
###
Abstract 54. “18F-PI-2620 Tau-PET in Progressive Supranuclear Palsy – A Multi-Center Evaluation,” Matthias Brendel, MD, Department of Nuclear Medicine, University of Munich, Munich, Germany; Henryk Barthel, MD, PhD, and Michael Rullmann, University of Leipzig, Leipzig, Germany; Thilo Van Eimeren, MD, Uniklinik Köln, Cologne, Germany; Kenneth Marek, MD, David S. Russell, MD, PhD, and John P. Seibyl, MD, Institute for Neurodegenerative Disorders, New Haven, Connecticut, USA; Leonie Beyer, MD, Department of Nuclear Medicine, University Hospital, Munich, Germany; Mengmeng Song, University of Munich, Munich, Germany; Carla Palleis, Neurology, University of Munich, Munich, Germany; Jochen Hammes, MD, Department of Nuclear Medicine, University Hospital Cologne, Cologne Germany; Dorothee Saur and Jost-Julian Rumpf, Neurology, University of Leipzig, Leipzig, Germany; Matthias Schroeter, MPI Human Cognitive & Brain Sciences, Leipzig, Germany; Andreas Schildan, PhD, and Osama Sabri, MD, PhD, University Hospital Leipzig, Leipzig, Germany; Marianne Patt, PhD, Department of Nuclear Medicine, Leipzig, Germany; Jennifer Madonia, Invicro, New Haven, Connecticut, USA; Andrew Stephens, Life Molecular Imaging GmbH, Berlin, Germany; Sigrun Roeber, Neuropathology, University of Munich, Munich, Germany; Johannes Levin, MD, Neurology Department Ludwig-Maximilians-University, Munich, Germany; Joseph Classen, Neurology, University of Leipzig, Leipzig, Germany; Guenter Hoeglinger, Neurology, Technical University of Munich, Munich, Germany; Peter A. Bartenstein, MD, Klinik und Poliklinik für Nuklearmedizin, Munich, Germany; and Alexander E. Drzezga, University Hospital of Cologne, Cologne, Germany. SNMMI’s 66TH Annual Meeting, June 22-25, 2019, Anaheim, CA.
Media Contact
David Harrison
[email protected]




