Researchers from Osaka University link the activity of water splitting catalysts to easy-to-measure parameters to enable rapid screening of clean energy generating materials
Credit: Osaka University
Osaka, Japan – Photocatalysts that harness light energy and use it to split water into hydrogen and oxygen attract significant scholarly attention, owing to the appeal of hydrogen as a potential clean energy source. However, the optimization of photocatalyst candidate materials usually requires a considerable time investment. Now, researchers at Osaka University have demonstrated a link between easy-to-measure quantities and catalyst performance that could provide a rapid evaluation method.
The conversion of light energy to chemical energy using photocatalysts has been widely reported; however, the continual optimization of photocatalytic materials is critical for their successful application. The properties of photocatalysts, including their surface area, crystallinity, and various electronic features, affect their activity. These properties can be influenced by the techniques and specific conditions used to prepare them, thus leading to a broad range of materials that could be evaluated.
Experiment setup and test of every generated material is a time-consuming step in the development process that has yet to be accelerated–until now. In a report published in ACS Energy Letters, Osaka researchers have shown the relationship between time-resolved microwave conductivity (TRMC) measurements and the photocatalytic performance of semiconductor materials. TRMC is a facile process that allows photocatalysts to be evaluated in powder form, which leads to significantly higher throughput.
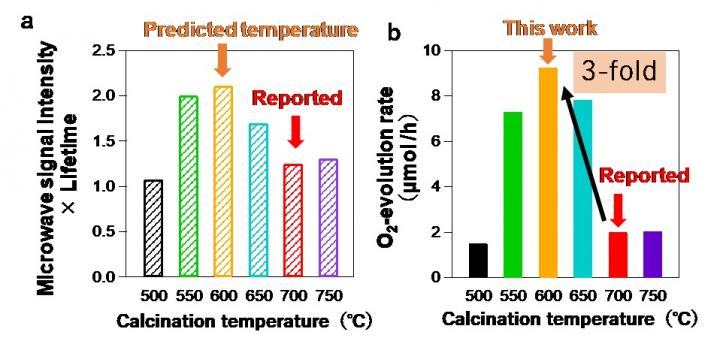
“We have been able to show that the oxygen evolution rate of a photocatalyst–which is a measure of activity–can be determined from the photoconductivity and the half-lifetime determined by TRMC,” explains study lead author Hajime Suzuki. “Applying this relationship to materials makes evaluating their potential much more efficient.”
The researchers used their findings to determine the optimum processing temperature for a material that had not been extensively studied, PbBiO2Cl, and were able to produce an analogue that had an apparent quantum efficiency of 3%–3 times higher than had been achieved in previous studies using higher processing temperatures.
“We hope that the principles of our findings can be widely applied to improve the efficiency and ease of screening materials, finding candidates, and choosing synthesis conditions,” study corresponding author Akinori Saeki explains. “In terms of the broader picture, high throughput processes could accelerate the development of cleaner energy solutions.”
###
The article, “Photoconductivity?Lifetime Product Correlates Well with the Photocatalytic Activity of Oxyhalides Bi4TaO8Cl and PbBiO2Cl: An Approach to Boost Their O2 Evolution Rates” was published in ACS Energy Letters at DOI: 10.1021/acsenergylett.9b00793.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




