Credit: Osaka University
Osaka, Japan – Polypropylene (PP) is everywhere, being one of the most widely used plastics in human life. A versatile material, its naturally inert surface can be modified for specific applications. Researchers at Osaka University have now developed a convenient light-driven process for oxidizing PP without harmful waste.
As reported in ChemComm, the process uses radicals to make the plastic react. The surface of PP bristles with methyl groups (-CH3), which constitute the side chains of the polymer. The strong C-H bonds in methyl groups make PP an unreactive material, which for many purposes is exactly what is needed. However, these bonds can be cleaved by the highly reactive chlorine dioxide radical, ClO2* .
“In applications like printing and medical materials, plastics must be surface-modified,” explains study co-author Tsuyoshi Inoue. “Oxidizing C-H bonds is a textbook case in organic chemistry. With polymers, however, the risk is that anything strong enough to do this may also break the C-C bonds of the main chain, ripping the polymer apart. Luckily, the ClO2* radical is selective to react the side chain.”
The highly reactive radical is easily made by mixing sodium chlorite and hydrochloric acid. It then just needs to be photochemically activated–for this, the Osaka team chose an LED lamp as the light source. The activated ClO2* now splits into Cl* , which whips off an H atom from the side chain of PP; and O2, which marches in afterward to oxidize the exposed -CH2* group.
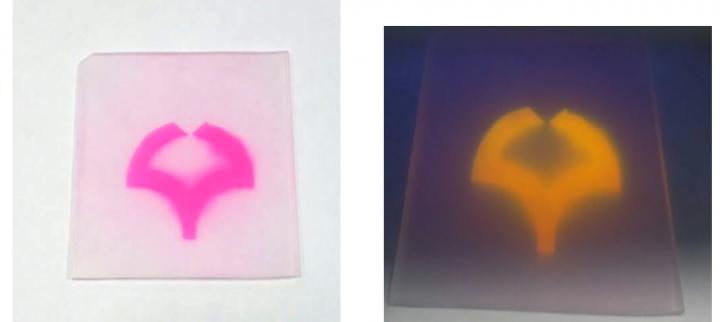
As a result, while the bulk polymer remains intact, the surface now bears a multitude of carboxylic acid groups (-CO2H), with major effects on the chemical reactivity. For example, the colorless plastic can now be stained with cationic dyes, such as Rhodamine B or Brilliant Green, which react with the anionic carboxylate ions. The originally water-repellent surface also becomes more hydrophilic.
“The reaction actually proved to be doubly selective for our purposes,” says lead author Kei Ohkubo. “Not only did it cleave the C-H instead of C-C bonds, it specifically oxidized those on the side chain, even though they are stronger than those on the main chain. This is because the oxidation step, involving O2, is most favorable when the target for oxidation is CH2* .”
Previous methods for oxidizing olefinic polymers such as PP and polyethylene were either poorly controlled or highly polluting. The new process is thus the first clean and convenient solution to this problem, and may prove to be a valuable industrial tool in the customization of synthetic plastics.
###
The article, “Photochemical C-H oxygenation of side-chain methyl groups in polypropylene with chlorine dioxide,” was published in ChemComm at DOI:10.1039/c9cc01037h.
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




