Two distinct physical mechanisms identified for how simple foams collapse
Credit: Rei Kurita
Tokyo, Japan – Researchers from Tokyo Metropolitan University have successfully found two distinct mechanisms by which foams can collapse, yielding insight into the prevention/acceleration of foam rupture in industrial materials e.g. foods, cosmetics, insulation, stored chemicals. When a bubble breaks, they found that a collapse event propagates via impact with the receding film and tiny scattered droplets breaking other bubbles. Identifying which mechanism is dominant in different foams may help tailor them to specific applications.
Foams play a key role in a wide range of industrial products, from foods, beverages, pharmaceuticals, cleaning products and cosmetics to material applications such as building insulation, aircraft interiors and flame-retardant barriers. They might also be an unwanted property of a product of e.g. frothing in stored chemicals during transit. From a scientific perspective, they also constitute a unique form of matter, a fine balance between the complex network of forces acting on the liquid film network that makes up its structure and the pressure of the gas trapped inside: understanding how foams behave may yield new physical insights as well as better ways to use them.
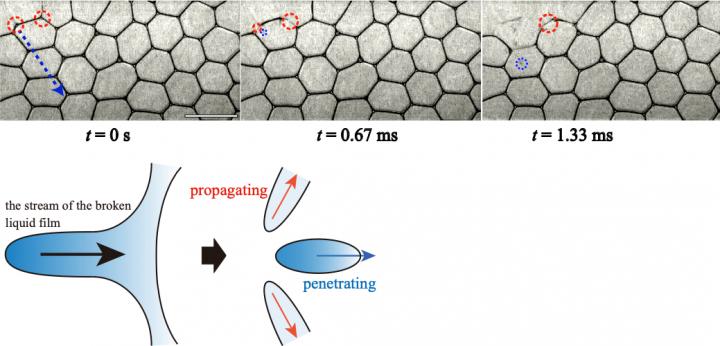
Naoya Yanagisawa and Associate Professor Rei Kurita set out to observe how foams collapse. They took a solution of water, glycerol and a common surfactant (a film-stabilizing agent) and created a two-dimensional foam squashed between two pieces of glass. Using an ultra-fast camera and a needle, they were able to controllably break a bubble at the edge of the foam raft and observe “collective bubble collapse” (CBC). They identified two distinct ways in which the breakage of one bubble at the edge led to a cascade of breakage events around it, a “propagating” mode due to the film of the broken bubble being absorbed into surrounding liquid film, and a “penetrating” mode due to droplets being released from the rupture event flying away and breaking other bubbles.
As the investigators changed the amount of water in the film, they identified several key trends in how the bubbles reacted at a microscopic level. For example, they found that more liquid in the foam led to the release of slower droplets, unable to penetrate surrounding films. This was correlated with a drastic drop in the number of bubbles collapsed; CBCs were thus crucially underpinned by the “penetrating” mode of collapse. Droplet speed was determined by the speed at which the film receded; this “streaming velocity” was found to be proportional to the osmotic pressure of the film i.e. the pressure at which a liquid brought into contact with the foam is driven into the film network. The team showed that the Navier-Stokes equations, key relations describing how fluids behave over time, could be used to explain these trends.
A key finding was that changing the viscosity of the fluid did not lead to a significant change in the number of bubbles broken. Methods to stabilize foams commonly rely on changing the viscosity, yet the team’s findings clearly show how both the number of bubbles collapsed and the velocity of the receding film are unaffected. Coupled with the dominant role played by the “penetrating” mode, future strategies to prevent foam collapse may instead focus on e.g. combining multiple surfactants to make the film more resistant to droplet impact.
###
This was work supported by a JSPS KAKENHI Grant-in-Aid for Scientific Research (B). The study has been published online in the journal Scientific Reports.
Media Contact
Go Totsukawa
[email protected]
Related Journal Article
http://dx.




