Credit: Kosack et al., 2019, Cancer Cell 35, 1-5, January 14, 2019 © 2018 Published by Elsevier Inc. DOI: 10.1016/j.ccell.2018.11.018.
Tumors usually grow exclusively in the organism where their cell of origin derives from. The same applies for human cancers: apart from some rare cases, like the accidental transmission by a cut during surgery, there are no reports of contagious cancer cells. A multitude of molecular safety measures of the immune system are? responsible for rejecting and destroying any foreign tissue.
An exception to this nearly universal rule exists among Tasmanian devils, the world’s largest living carnivorous marsupial. For two decades, a deadly facial tumor has been spreading at a rapid pace among the animals and has killed, according to current estimates around 90 percent of the wild population. Peculiarly, the cancer cells are transmitted from one Tasmanian devil to the other by bites. All collected tumor samples are genetically nearly identical and derive presumably from a single cell of origin.
How this cancer became transmissible and by what means it escapes the immune system of its otherwise healthy hosts puzzled scientists since the discovery of the mysterious disease. Researchers from CeMM and the Vienna University of Veterinary Medicine now solved an important part of this puzzle. In their latest study published in Cancer Cell (DOI: 10.1016/j.ccell.2018.11.018.), the research groups of Andreas Bergthaler, Principal Investigator at CeMM, and Richard Moriggl, Head of the Ludwig Boltzmann Institute for Cancer Research and Professor for Functional Cancer Genomics at the Vienna University of Veterinary Medicine and the Medical University of Vienna, revealed molecular mechanisms that are crucial for the transmissibility of the tumor.
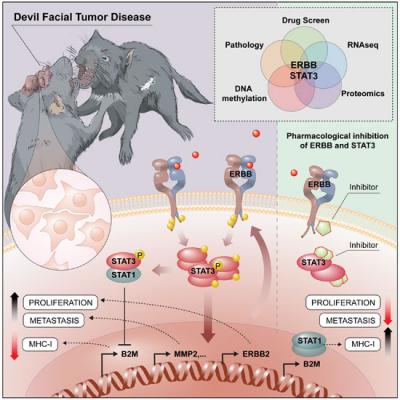
The scientists found that receptor molecules on the surface of the cancer cells, so-called ERBB receptors, show massively increased activity. Those receptors trigger a biochemical chain reaction within the cells that eventually activates STAT3 proteins, transcription factors that alter the cell’s genetic program. The result is an extensive rebuild of the cell: The number of molecules serving as identification for the immune system are reduced, while at the same time proliferation is accelerated and factors for metastasis of the tumor cells are produced.
“Our experiments show for the first time that the excessive activation of ERBB receptors and STAT3 proteins play a key role in the transmissibility of the Tasmanian devil´s facial tumor”, Lindsay Kosack, team member of Bergthaler’s group at CeMM and co-first author, explains. “Above that, we showed in further experiments that the inhibition of ERBB receptors with a drug can selectively kill the cancer cells. This could play an important role for the treatment of the disease, before the Tasmanian devil becomes extinct”.
The study, conducted in collaboration with the Universities of Cambridge, Southampton, Toronto and Tasmania, is not only a contribution to preserve this marsupial species. “99.1 percent of the Tasmanian devil’s STAT3 are identical to the human protein. And many of the genes that are activated by STAT3 in the animals are also active in human cancers”, Andreas Bergthaler says. “The biological principles of invasion of new tissues are also crucial for non-transmissible tumors, especially cancer metastases. Scientific aspects of cancer, contagious diseases and inflammatory processes can be studied with this rare phenomenon”.
However, it is unlikely – although not impossible – that a human cancer becomes transmissible, Bergthaler emphasizes. “Apart from the molecular mechanisms that would need to evolve are humans genetically much more diverse and resistant than the isolated population of the Tasmanian devils. The aggressive biting behavior of the animals also seems to play an important role in tumor transmission. Nevertheless a better molecular understanding of this rare disease can provide valuable insights on fundamental biological mechanisms of cancer development”.
###
Videos: https:/
Keyword: devil
https:/
(Video abstract at the Cancer Cell website will be made publicly available once the embargo lifts)
The study „The ERBB-STAT3 Axis Drives Tasmanian Devil Facial Tumor Disease” was published in Cancer Cell, on 14 January 2019, DOI: 10.1016/j.ccell.2018.11.018.
Authors: Lindsay Kosack*, Bettina Wingelhofer*, Alexandra Popa*, Anna Orlova*, Benedikt Agerer, Bojan Vilagos, Peter Majek, Katja Parapatics, Alexander Lercher, Anna Ringler, Johanna Klughammer, Mark Smyth, Kseniya Khamina, Hatoon Baazim, Elvin D. de Araujo, David A. Rosa, Jisung Park, Gary Tin, Siawash Ahmar, Patrick T. Gunning, Christoph Bock, Hannah V. Siddle, Gregory M. Woods, Stefan Kubicek, Elisabeth P. Murchison, Keiryn L. Bennett, Richard Moriggl*, and Andreas Bergthaler*
*These authors contributed equally.
The study was funded by the Austrian Academy of Sciences, the Austrian Science Fund (FWF), the European Research Council (ERC) and the Austrian Research Promotion Agency (FFG).
Andreas Bergthaler studied Veterinary Medicine in Vienna. He undertook graduate and postgraduate research with Hans Hengartner and Nobel Laureate Rolf Zinkernagel at the University of Zurich and ETH Zurich followed by postdoctoral positions with Daniel Pinschewer at the University of Geneva and with Alan Aderem at the Institute for Systems Biology in Seattle. He joined CeMM in 2011 and holds an ERC Starting Grant.
The mission of CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences is to achieve maximum scientific innovation in molecular medicine to improve healthcare. At CeMM, an international and creative team of scientists and medical doctors pursues free-minded basic life science research in a large and vibrant hospital environment of outstanding medical tradition and practice. CeMM’s research is based on post-genomic technologies and focuses on societally important diseases, such as immune disorders and infections, cancer and metabolic disorders. CeMM operates in a unique mode of super-cooperation, connecting biology with medicine, experiments with computation, discovery with translation, and science with society and the arts. The goal of CeMM is to pioneer the science that nurtures the precise, personalized, predictive and preventive medicine of the future. CeMM trains a modern blend of biomedical scientists and is located at the campus of the General Hospital and the Medical University of Vienna.
Media Contact
Eva Schweng
[email protected]
0043-140-160-70051
Related Journal Article
http://dx.




