Two catheters originally developed by Temple cardiologist Dr. Riyaz Bashir receive premarket clearance by FDA for treatment of blood clots
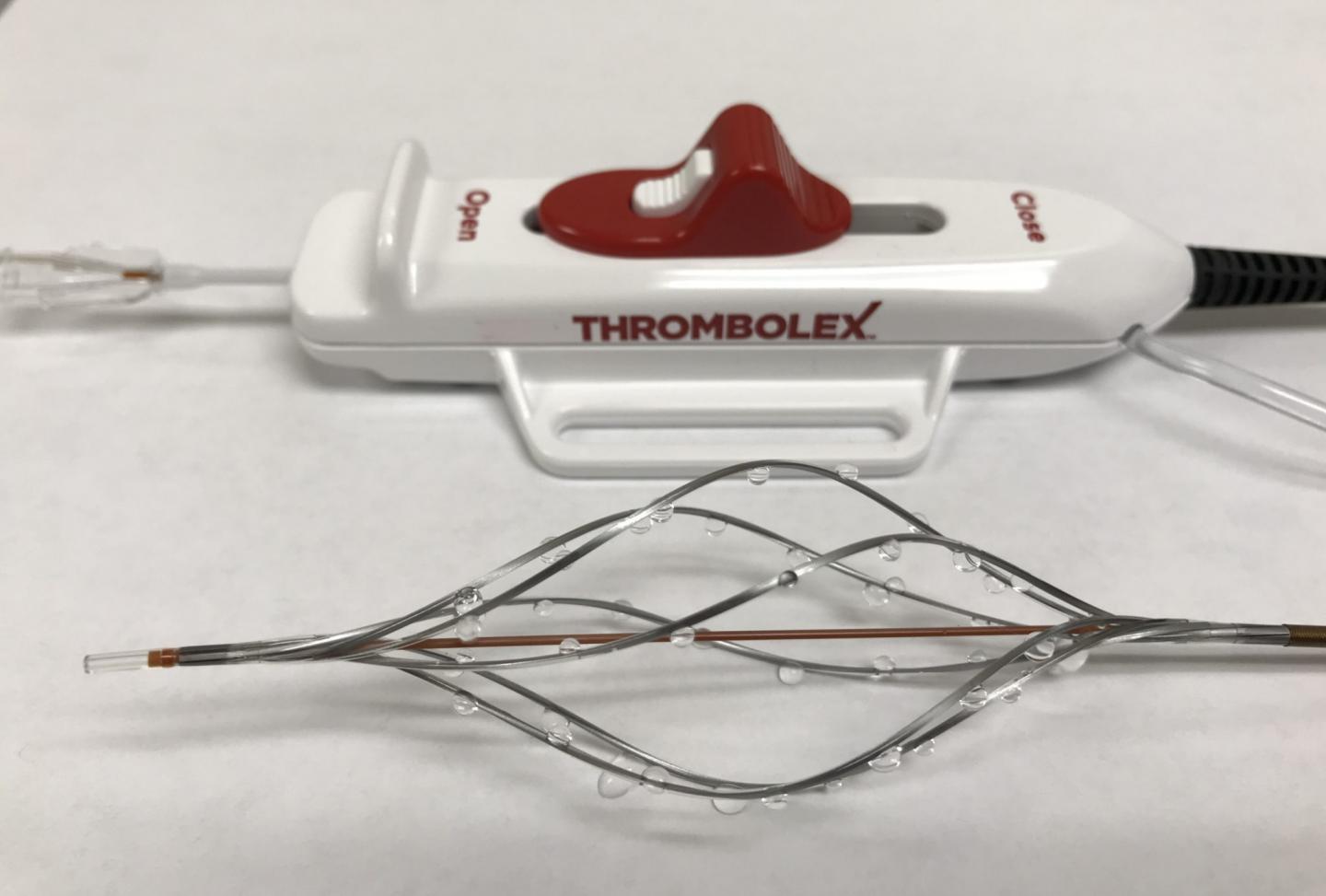
Credit: Thrombolex, Inc.
(Philadelphia, PA) – The U.S. Food and Drug Administration (FDA) has granted premarket notification clearance for two catheters invented by Riyaz Bashir, MD, FACC, RVT, Professor of Medicine at the Lewis Katz School of Medicine at Temple University (LKSOM) and Director of Vascular and Endovascular Medicine at Temple University Hospital, and Nicholas Green, Director of Research & Development at Thrombolex, Inc. Thrombolex is a company founded in partnership with Temple University to develop these catheter-based clot dissolving devices. This clearance now allows Thrombolex to commercialize the catheters.
The Bashir™ Endovascular Catheter (BEC) is cleared for the controlled and selective infusion of fluids, including clot-dissolving medications, into the veins and arteries of the peripheral vasculature. The BEC is unique because it’s the only catheter of its kind that, once advanced into the clot, can be expanded by the physician into six expandable mini-catheters to deliver medications in precise locations throughout the cross section of the clot.
The Bashir N-X™ Endovascular Catheter (BEC N-X) is cleared for the controlled and selective infusion of fluids chosen by the physician into both the peripheral and pulmonary vasculature, which is comprised of the blood vessels of the lungs. Unlike the BEC, the BEC N-X is not expandable.
“My inspiration for the BEC platform technology was to develop a device that I hoped would provide better treatment outcomes by rapid restoration of blood flow through the blood clot thereby enhancing the breakdown of the clot,” said Dr. Bashir. “Acute Venous Thromboembolic (VTE) disease, which is marked by blood clots that start in a vein – often in the deep veins of the leg, groin or arm – and can break off and travel to the lungs causing a pulmonary embolism, has become a significant public health concern in the U.S. Approximately 900,000 patients have been diagnosed with VTE and it causes up to 100,000 deaths each year, according to the CDC.”
“Our dedicated team is proud to have received FDA clearance for these two unique catheter-directed thrombolysis products to be used in the treatment of patients suffering from acute VTE disorders,” said Marvin Woodall, Chairman and CEO and co-founder of Thrombolex.
Thrombolex has also received FDA approval to begin a multicenter early feasibility study in the clinical setting to evaluate the safety and feasibility of the Bashir™ Endovascular Catheter in the treatment of acute pulmonary embolism.
###
Editor’s Note: Dr. Bashir is a co-founder and has equity interest in Thrombolex, Inc. a medical device company developing interventional catheter-based therapies for the rapid and effective treatment of acute venous thromboembolic disorders. Temple University also holds a financial interest in Thrombolex, Inc., pursuant to the license granted to Thrombolex for the University’s interest in the patent filed for the experimental catheter device developed by Dr. Bashir and Nicholas Green.
About Temple Health
Temple University Health System (TUHS) is a $2.1 billion academic health system dedicated to providing access to quality patient care and supporting excellence in medical education and research. The Health System consists of Temple University Hospital (TUH), ranked among the “Best Hospitals” in the region by U.S. News & World Report; TUH-Episcopal Campus; TUH-Northeastern Campus; Fox Chase Cancer Center, an NCI-designated comprehensive cancer center; Jeanes Hospital, a community-based hospital offering medical, surgical and emergency services; Temple Transport Team, a ground and air-ambulance company; and Temple Physicians, Inc., a network of community-based specialty and primary-care physician practices. TUHS is affiliated with the Lewis Katz School of Medicine at Temple University, and Temple University Physicians, which is Temple Health’s physician practice plan comprised of more than 500 full-time and part-time academic physicians in 20 clinical departments.
The Lewis Katz School of Medicine (LKSOM), established in 1901, is one of the nation’s leading medical schools. Each year, the School of Medicine educates more than 800 medical students and approximately 240 graduate students. Based on its level of funding from the National Institutes of Health, the Katz School of Medicine is the second-highest ranked medical school in Philadelphia and the third-highest in the Commonwealth of Pennsylvania. According to U.S. News & World Report, LKSOM is among the top 10 most applied-to medical schools in the nation.
Temple Health refers to the health, education and research activities carried out by the affiliates of Temple University Health System (TUHS) and by the Katz School of Medicine. TUHS neither provides nor controls the provision of health care. All health care is provided by its member organizations or independent health care providers affiliated with TUHS member organizations. Each TUHS member organization is owned and operated pursuant to its governing documents.
It is the policy of Temple University Hospital, Inc. that there shall be no exclusion from, or participation in, and no one denied the benefits of, the delivery of quality medical care on the basis of race, ethnicity, religion, sexual orientation, gender, gender identity/expression, disability, age, ancestry, color, national origin, physical ability, level of education, or source of payment.
About Thrombolex, Inc.
Founded in 2016, Thrombolex, Inc. is engaged in the design, development, manufacture and distribution of innovative endovascular catheters used in interventional procedures, particularly in catheter-directed thrombolysis of large thrombus in patients affected by acute VTE disorders. Our vision is to orchestrate the medical profession’s identified unmet needs with our unique product development capabilities. Our goal is to create a continuous stream of new products based on the original BASHIR™ platform technology, which we hope will improve the treatment outcomes for patients suffering from acute VTE. Please visit http://www.
Media Contact
Jeremy Walter
[email protected]
Original Source
https:/




