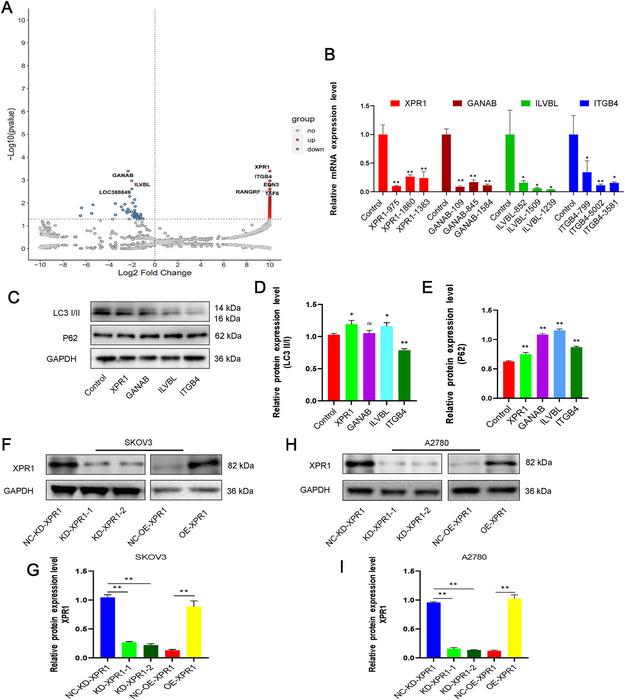
A groundbreaking study published in the prestigious journal Genes & Diseases has unveiled a pivotal role for the gene XPR1 in the progression of ovarian cancer, illuminating new molecular pathways that could revolutionize therapeutic strategies against this formidable malignancy. Scientists at Chongqing Medical University have identified XPR1 as a key regulator of autophagy—a cellular degradation and recycling process—and as a modulator of major histocompatibility complex class I (MHC-I) expression. The discovery not only sheds light on the molecular underpinnings of ovarian cancer aggressiveness but also suggests innovative avenues to overcome resistance to contemporary immunotherapies.
Ovarian cancer remains one of the deadliest gynecological cancers globally, primarily due to its late diagnosis, rapid metastasis, and frequent resistance to immune checkpoint blockade therapies such as PD-1 and CTLA-4 inhibitors. In this context, elucidating the molecular factors that govern tumor growth and immune evasion is vital. The research team harnessed a CRISPR-Cas9 library screening, an advanced gene-editing technology, to systematically investigate candidate genes influencing autophagy in ovarian cancer models. This approach pinpointed XPR1 as a previously underappreciated gene whose elevated expression correlates positively with ovarian cancer severity.
Detailed pathological evaluations revealed that XPR1 expression is markedly increased in carcinoma tissues compared to normal ovarian epithelium. Importantly, this heightened expression aligns with advanced tumor stages and is inversely correlated with patient overall survival and progression-free survival metrics. These clinical correlations indicate that XPR1 does not merely associate with but likely actively drives tumor malignancy.
.adsslot_fUSDOW37vC{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_fUSDOW37vC{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_fUSDOW37vC{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
At the cellular level, experimental silencing of XPR1 via RNA interference techniques significantly impaired ovarian cancer cell proliferation and migration, indicating its functional necessity for cancer progression. Conversely, forced overexpression of XPR1 augmented proliferative and metastatic capabilities in vitro. This bidirectional manipulation confirms the oncogenic phenotype driven by XPR1 and positions it as a compelling therapeutic target.
Mechanistic investigations revealed that XPR1 interacts physically with lysosomal-associated membrane protein 1 (LAMP1), a crucial component of lysosomal membranes involved in autophagy. This interaction modulates autophagy flux, particularly by dampening autophagic activity during the early and lysosomal stages. Autophagy, while traditionally considered a cell survival mechanism, has complex roles in cancer biology, capable of both suppressing and facilitating tumor growth depending on context. XPR1’s regulation of autophagy flux via LAMP1 suppresses lysosome formation and autophagic degradation processes, thereby enhancing ovarian cancer cell survival.
Further probing uncovered that XPR1’s modulation of autophagy operates predominantly through the PI3K/Akt/mTOR signaling pathway, a well-known axis controlling cell growth and metabolism. By stimulating this pathway, XPR1 inhibits autophagy, thereby providing cancer cells a survival advantage under metabolic and environmental stress. This insight not only advances understanding of ovarian cancer cell biology but also links XPR1 activity to broad oncogenic signaling networks.
Strikingly, the study also revealed a novel role for XPR1 in immune evasion. Expression levels of MHC-I molecules on the tumor cell surface are critical for recognition and cytotoxic attack by CD8+ T lymphocytes. XPR1-mediated autophagy regulation appears to control the degradation of MHC-I proteins, thus diminishing antigen presentation and enabling tumor cells to escape immune surveillance. Therapeutic silencing of XPR1 increased MHC-I presence, implying enhanced immunogenicity.
Exploiting this vulnerability, the investigators combined XPR1 silencing with chloroquine, a known autophagy inhibitor, in mouse models of ovarian cancer. This combinatorial treatment synergistically increased MHC-I expression, revived anti-tumor immune responses, and led to significant tumor growth suppression. These findings suggest that blocking autophagy to sustain MHC-I surface levels may potentiate immune checkpoint inhibitor therapies, potentially overcoming their frequent failure in ovarian cancer.
The implications of these results are profound. Targeting XPR1 directly or indirectly through autophagy modulation holds promise as an adjuvant or alternative approach in treating ovarian cancers that are refractory to current immunotherapies. Given the association of XPR1 with both cancer cell intrinsic survival pathways and immune evasion mechanisms, therapeutic strategies disrupting its activity could strike a dual blow to tumor progression.
Moreover, this research opens a new line of inquiry into how autophagy controls antigen presentation beyond ovarian cancer, possibly extending to other malignancies notorious for immune escape and therapy resistance. Future investigations might delineate combinatorial regimens pairing autophagy inhibitors with checkpoint blockade drugs, optimizing treatment efficacy and patient outcomes.
Notably, the study emphasizes the utility of CRISPR-Cas9-based functional genomics in identifying actionable cancer drivers and refining molecular targeted therapies. The methodological rigor and translational relevance underscore the potential for rapid preclinical development of XPR1 inhibitors or RNA-based therapeutics.
In summary, the identification of XPR1 as a key orchestrator of autophagy and MHC-I regulation establishes it as a novel molecular nexus in ovarian cancer pathogenesis. By bridging tumor biology with immune modulation, this discovery advances the frontier of personalized cancer therapy and offers hope for improving prognosis in a malignancy that urgently requires new therapeutic paradigms.
Subject of Research: Role of XPR1 in ovarian cancer growth and immune evasion through autophagy regulation
Article Title: XPR1 promotes ovarian cancer growth and regulates MHC-I through autophagy
News Publication Date: 2024 (specific date not provided)
References: Hui Wang, Xiaodong Luo, Bo Yang, Furong Tang, Xingwei Jiang, Hongtao Zhu, Jianguo Hu, Genes & Diseases, Volume 12, Issue 5, 2025, Article 101507, DOI: 10.1016/j.gendis.2024.101507
Image Credits: Genes & Diseases
Keywords: Cancer genetics, ovarian cancer, XPR1, autophagy, MHC-I, immune evasion, lysosomal function, PI3K/Akt/mTOR pathway, CRISPR-Cas9 screening, immunotherapy resistance
Tags: autophagy in cancercarcinoma tissue analysisCRISPR-Cas9 gene editinggynecological cancer researchImmune Evasion MechanismsMHC-I expression modulationmolecular pathways in cancerovarian cancer progressionovarian cancer therapeutic strategiesresistance to immunotherapytumor growth factorsXPR1 gene regulation