Credit: WFIRM
WINSTON-SALEM, N.C. – May 29, 2018 – Wake Forest Institute for Regenerative Medicine (WFIRM) scientists have developed a 3-D brain organoid that could have potential applications in drug discovery and disease modeling. This is the first engineered tissue equivalent to closely resemble normal human brain anatomy, containing all six major cell types found in normal organs including, neurons and immune cells.
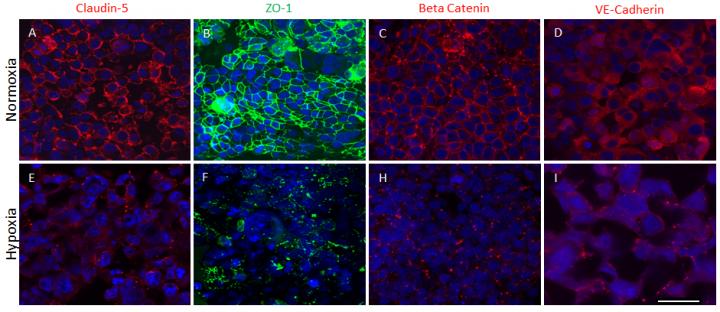
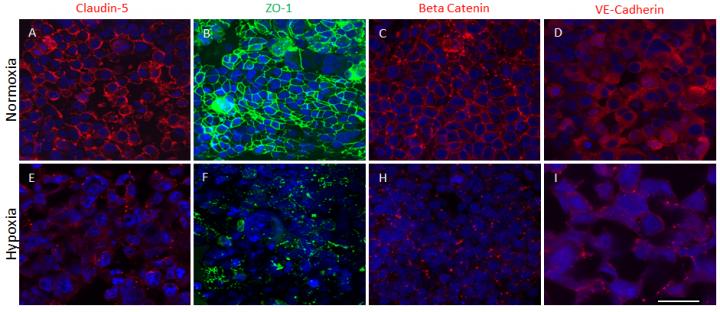
In a study published this month in Scientific Reports, the researchers report that their advanced 3-D organoids promote the formation of a fully cell-based, natural and functional barrier – the blood brain barrier – that mimics normal human anatomy.
The blood brain barrier is a semipermeable membrane that separates the circulating blood from the brain, protecting it from foreign substances that could cause injury. This development is important because the model can help to further understanding of disease mechanisms at the blood brain barrier, the passage of drugs through the barrier, and the effects of drugs once they cross the barrier.
"The shortage of effective therapies and low success rate of investigational drugs are due in part because we do not have a human-like tissue models for testing," said senior author Anthony Atala, M.D., director of WFIRM. "The development of tissue engineered 3D brain tissue equivalents such as these can help advance the science toward better treatments and improve patients' lives."
The development of the model opens the door to speedier drug discovery and screening, both for neurological conditions and for diseases like HIV where pathogens hide in the brain and avoid current treatments that cannot cross the blood brain barrier. It may also allow for disease modeling of neurological conditions such as Alzheimer's disease, multiple sclerosis and Parkinson's disease so that researchers can better understand their pathways and progression.
Thus far the researchers have used the brain organoids to mimic strokes in order to measure impairment of the blood brain barrier and have successfully tested the model's permeability with large and small molecules.
"Using an engineered tissue model provides a platform that can be used to understand the fundamental principles at play with the blood brain barrier and its function, as well as the effects of chemical substances that cross it," said Goodwell Nzou, a Ph.D. candidate at WFIRM who co-authored the paper.
###
Co-authors include: John Jackson, Ph.D., and Sean Murphy, Ph.D., WFIRM faculty; Elizabeth Wicks, Stephanie Seale, C.H. Sane, A. Chen, all students who participated in the Summer Scholars program; and Robert Wicks, M.D., Wake Forest Baptist, Neurological Surgery.
The authors declare no competing interests and there was no external funding.
Media contact: Bonnie Davis, [email protected], 336-713-1597.
Media Contact
Bonnie Davis
[email protected]
336-713-1597
@wakehealth
http://www.wfubmc.edu
Related Journal Article
http://dx.doi.org/10.1038/s41598-018-25603-5