Kanazawa University researchers design a radical reaction to build highly functionalized ketones, installing two new carbon-carbon bonds at adjacent positions using a non-metal catalyst
Credit: Kanazawa University
Kanazawa, Japan – Organic chemists are molecular architects, designing sophisticated structures. As the molecules used in science and medicine become ever more complex, new tools are needed to piece together the building blocks. Now, a Japanese team at Kanazawa University has developed a reaction that links up three components at once using free radical chemistry.
Carbon-carbon double bonds, known as alkenes, are more reactive than single bonds. This makes them useful for connecting different groups: if the double bond is broken, two new molecules can be added at either end, bridged together by the now single-bonded former alkene.
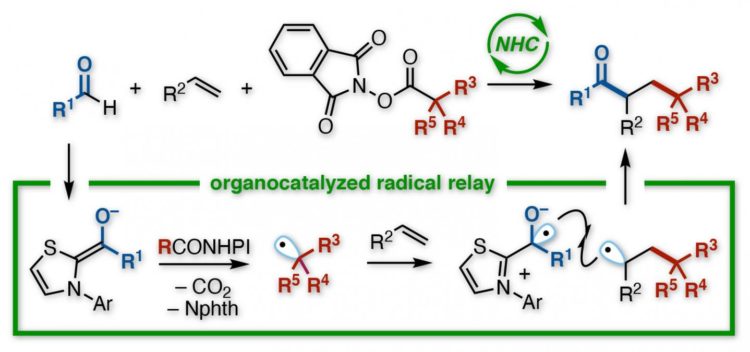
The Kanazawa team looked at ways to achieve this transformation using aldehydes, which contain a double bond between oxygen and carbon, as one of the two molecules grafted onto an alkene. As reported in the Journal of the American Chemical Society, the goal was to create highly functionalized ketones, an important family of compounds in organic synthesis.
Their chosen method was a reaction known, evocatively enough, as a radical relay. Containing an unpaired electron, and thus a half-empty orbital, radicals (or free radicals) tend to be voraciously reactive. So reactive, indeed, that they cannot be stored, but must be generated in-situ by whipping off an electron from somewhere during a reaction, sparking a cascade of bond-breaking and making.
Chemists can make radicals in various ways, but the Kanazawa team wanted to road-test oraganocatalyst, termed an N-heterocyclic carbene (NHC). With an aldehyde as one reactant, and a functionalized redox ester as the other, the NHC triggered their transformation into radicals. Previous studies hinted that both radicals would react faster with an alkene than with each other.
“The challenge with radicals is that, being so unstable, they often react with themselves,” says study co-author Kazunori Nagao. “Then you get either your starting materials back, or a side-product, instead of the target. We minimized this here. In fact, the reaction proceeded as a relay–first the alkyl radical added to one carbon of the alkene, and then the acyl radical added to the other.”
The resulting compound had a ketone and an ester-derived group bonded to two adjacent carbons, in a so-called vicinal arrangement. Although direct reaction between the different radicals also occurred–giving an unwanted two-component product, instead of the alkene-bridged three-component target–it was minor. Furthermore, a wide range of starting materials were compatible with the process.
“Two crucial points are worth mentioning,” says Hirohisa Ohmiya, corresponding author of the study. “First, unlike some reactions, ours is free of metal catalysts and does not need a light source to produce radicals, making it green and versatile. Second, the NHC precisely controls the relay, so we can be selective in the positions of functional groups. We are now working on an asymmetric version for chiral products.”
###
Media Contact
Tomoya Sato
[email protected]
81-762-645-076
Original Source
https:/
Related Journal Article
http://dx.