Research groups led by Prof. XU Tongwen and Prof. LI Xingya from the University of Science and Technology of China (USTC) have proposed the concept of total dehydration of ions, and prepared metal-organic framework (MOF) confined crown ether membranes, which solved the problem of precise separation of ions in complex systems. The research results were published in Science Advances.
Credit: ustc
Research groups led by Prof. XU Tongwen and Prof. LI Xingya from the University of Science and Technology of China (USTC) have proposed the concept of total dehydration of ions, and prepared metal-organic framework (MOF) confined crown ether membranes, which solved the problem of precise separation of ions in complex systems. The research results were published in Science Advances.
The efficient and precise separation of ions involves important chemical processes such as lithium extraction from salt lakes, seawater refining, and high-salt wastewater resource utilization. The ions in these systems have complex compositions, and their sizes are all at the angstrom level, which is a technical challenge that needs to be solved in the current chemical separation industry.
According to the team’s previous research results, the angstrom-scale membrane channels helped ions undergo partial dehydration and promoted ion transfer and sieving. In order to further enhance the flux and selectivity of monovalent ions, the researchers continued the work.
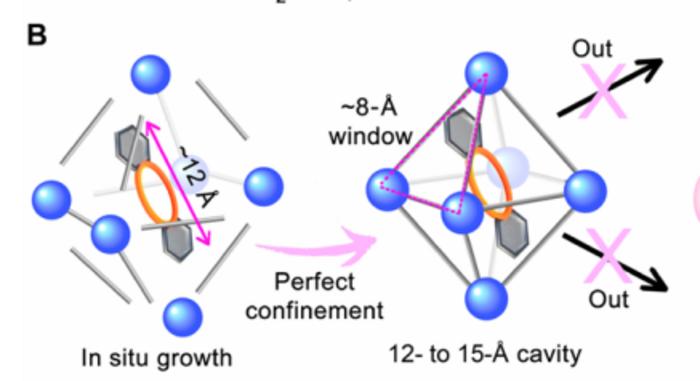
Researchers aimed to realize the in-situ growth of MOF confinement crown ether (CE@UiO-66) membranes under mild conditions (30 ℃), where there was “perfect confinement” of crown ether molecules by utilizing the window-cavity structure of UiO-66.
The UiO-66 had a window of ~8 Å and a cavity of ~12-15 Å. Based on the size-matching effect, it was possible to restrict dibenzo-15-crown-5 (DB15C5) or dibenzo-18-crown-6 (DB18C6) with a molecular size of ~12 Å. The UiO-66 membrane had a window of ~8 Å and a cavity of ~12-15 Å.
Compared to pristine UiO-66 membranes, CE@UiO-66 membranes exhibited higher monovalent ion permeation rates and selectivity due to the synergistic effect of UiO-66 window size screening and crown ether interaction screening that allowed complete dehydration of monovalent ions.
In the concentration-driven binary ion separation system, the flux of monovalent cations (e.g., K+) and the selectivity of monovalent/divalent cations (e.g., K+/Mg2+) were in the range of 0.9 ~ 1.2 mol m2h-1 and 30 ~ 60, respectively.
First-principles calculations and molecular dynamics simulations showed that the monovalent ions were completely dehydrated when passing through the DB18C6@UiO-66 cavity, which prompted the rapid transfer of monovalent ions with a lower energy barrier (transfer energy barrier: K+< Na+< Li+< Mg2+), and the final ionic transmembrane rate was: K+> Na+> Li+> Mg2+.
The study provides theoretical guidance for the construction of precise separation membranes for ions in complex systems.
Journal
Science Advances
DOI
10.1126/sciadv.adn0944
Article Title
Perfect confinement of crown ethers in MOF membrane for complete dehydration and fast transport of monovalent ions
Article Publication Date
8-May-2024




