Professor HUANG Hanmin’s research team from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) proposed a new paradigm of metal electron-shuttle catalysis, which has been pioneeringly employed to achieve alkylative aminomethylation of unactivated alkene for the first time. Their work was published in Nature Catalysis on August 21st.
Credit: Image by RAO Changqing et al.
Professor HUANG Hanmin’s research team from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) proposed a new paradigm of metal electron-shuttle catalysis, which has been pioneeringly employed to achieve alkylative aminomethylation of unactivated alkene for the first time. Their work was published in Nature Catalysis on August 21st.
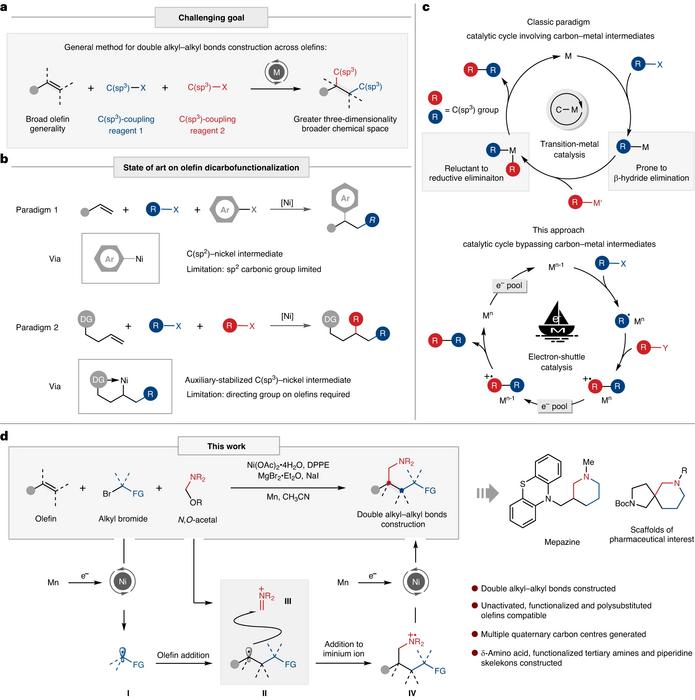
Transition metal-catalyzed carbon-carbon bond formation has been an integral part of contemporary organic chemistry research. However, the development of transition metal-catalyzed C(sp3)-C(sp3) bond formation has been relatively slow due to the generation of unstable alkyl-metal intermediates during the reaction, which lead to various side reactions, making it difficult to apply the classical catalytic paradigm to C(sp3)-C(sp3) bond formation.
The dicarbofunctionalization of alkenes, where two carbon functional groups are introduced at both ends of the alkene in a single operation, has gained vast attention for its ability to efficiently connect carbon atoms, thereby facilitating the construction of complex molecules. Existing researches are often limited to the introduction of C(sp2) functional groups, or they require the pre-installation of coordinating groups onto the alkenes to stabilize the alkyl-metal intermediates. This undeniably reduces the substrate applicability and step economy of the reaction.
To solve the challenges of alkene difunctionalization reactions, the research team, proposed a new paradigm of metal electron-shuttle catalysis. Specifically, they utilized a metal catalyst as an electron shuttle to initiate and quench radicals, thereby achieving the construction of multiple alkyl-alkyl bonds through radical-unsaturated bond addition, effectively avoiding the generation of unstable alkyl-metal intermediates.
In this study, the team employed a nickel catalyst as the electron shuttle and used N, O-acetals and alkyl halides as the alkylating agents to accomplish the dialkylation of unactivated alkenes. This method demonstrated excellent compatibility with simple alkenes, unactivated alkenes and polysubstituted alkenes. Additionally, various types of alkylating agents could be applied under such reaction conditions. Secondary amines and paraformaldehyde could also replace N, O-acetals in the four-component reaction, further broadening the scope of the reaction’s applications.
This reaction offers an efficient method for the synthesis of fluorinated and non-fluorinated δ-amino acids and other unnatural amino acid derivatives. Moreover, this reaction introduces various functional groups, which could further transform to produce more valuable complex molecules. For instance, by reducing and cyclizing the reaction products, one can swiftly construct piperidine compounds, which are prevalent in pharmaceutical molecules. Researchers synthesized the pharmaceutical molecule Mepazine and its corresponding fluorinated derivatives through this transformation strategy, demonstrating the practical value of the reaction.
Reviewers from Nature Catalysis highly appreciated the work, saying that “…the findings are sufficiently compelling…”,”this synthetically important paper represented an excellent work of radical-mediated difunctionalization reaction.” It’s worth emphasizing that this reaction not only offers significant synthetic applicability but also serves as an exemplar of the metal electron-shuttle catalysis paradigm, showcasing the promising prospects of this catalytic approach. Further research into the metal electron-shuttle catalysis will play a pivotal role in the development of drug and functional molecule synthesis.
Journal
Nature Catalysis
DOI
10.1038/s41929-023-01015-1
Article Title
Double alkyl–alkyl bond construction across alkenes enabled by nickel electron-shuttle catalysis
Article Publication Date
21-Aug-2023




