Adenosine triphosphate (ATP) is the energy currency of cells. It powers various cellular processes that require energy, including enzymatic reactions. ATP is synthesized with the help of an enzyme complex called F-type ATP synthase. This enzyme complex has a bidirectional functionality, working to synthesize ATP as well as hydrolyzing it, depending on environmental and cellular cues. ATP synthase consists of two rotating motors—F1 and F0. The F1 subcomplex is mainly composed of α, β, and γ subunits. During the hydrolysis of ATP, the F1-ATPase show rotational motion. Therefore, F1-ATPase is also known as the world’s smallest rotary biological molecule motor. However, the underlying mechanism of how ATP hydrolysis makes the molecule rotate remains unclear.
Credit: Dr. Tomoko Masaike
Adenosine triphosphate (ATP) is the energy currency of cells. It powers various cellular processes that require energy, including enzymatic reactions. ATP is synthesized with the help of an enzyme complex called F-type ATP synthase. This enzyme complex has a bidirectional functionality, working to synthesize ATP as well as hydrolyzing it, depending on environmental and cellular cues. ATP synthase consists of two rotating motors—F1 and F0. The F1 subcomplex is mainly composed of α, β, and γ subunits. During the hydrolysis of ATP, the F1-ATPase show rotational motion. Therefore, F1-ATPase is also known as the world’s smallest rotary biological molecule motor. However, the underlying mechanism of how ATP hydrolysis makes the molecule rotate remains unclear.
To address this knowledge gap, a team of researchers from Japan, led by Associate Professor Tomoko Masaike from Tokyo University of Science, set out to investigate the events behind the rotational mechanism of F1-ATPase in the thermophilic bacterium, Bacillus PS3. Elaborating on the objectives of their study, Dr. Masaike explains, “We wanted to clarify the mechanism by which F1-ATPase rotates the central shaft during ATP hydrolysis. We focused on clarifying the angle of rotation of the central shaft between the binding of the substrate ATP to the enzyme and the cleavage of its high-energy phosphate bond.” The study, which was undertaken in collaboration with Professor Takayuki Nishizaka from Gakushuin University and Yuh Hasimoto from Hamamatsu Photonics K.K., was made available online on 21 December 2022 and 7 February 2023 in print in Biophysical Journal.
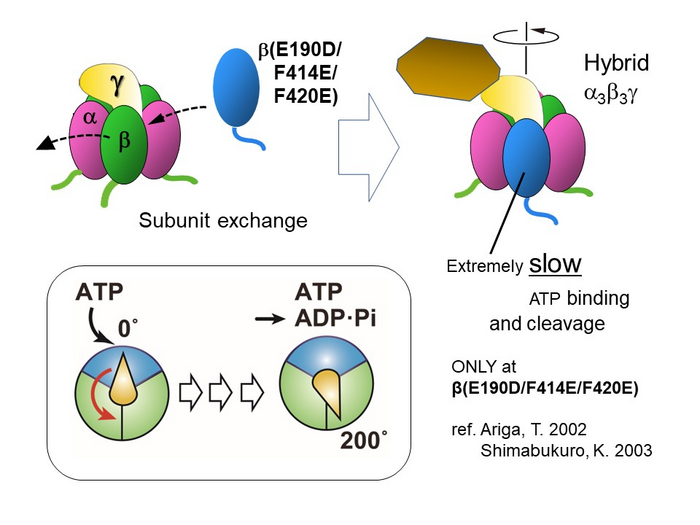
Previous investigations of the F1 subunits of Bacillus PS3 have established that ATP cleavage involves chemomechanical coupling, i.e., each rotational stepping motion is linked to a chemical reaction step. The angle of rotation between ATP binding and its cleavage at the same catalytic site has been previously estimated to be 200°. However, experimental evidence to substantiate this has so far been lacking. To address this, the researchers studied the rotation by creating a hybrid F1 using one mutant β and two wild type βs. Since the rate of both ATP cleavage and ATP binding was extremely slow in the mutant, the researchers could observe the pauses or dwells between rotational steps easily.
Upon performing a single-molecule rotation assay with varying concentrations of ATP, they could observe three sets of short and long dwells associated alternately with 80° and 40° intervals per revolution. To investigate the events associated with the dwells, the authors performed dwell-time analyses. The long pause before the 40° sub-step was independent of ATP concentration and was confirmed as the ‘catalytic dwell’—a pause in the rotation of the shaft due to ATP cleavage. Alternately, the short pause before the 80° step was clearly dependent on ATP concentration and thus identified as the ‘ATP-waiting dwell’ (pause to enable β subunit to bind ATP). “Upon investigating the rotation of the shaft, we could provide visible evidence through optical microscopy that the shaft angles at ATP-binding and cleaving events in Bacillus PS3 were 0° and 200°, respectively” says Hasimoto.
With this study, the authors have resolved a long-term debate over the ATP-cleavage shaft angle and established the chemomechanical correlation of ATPase function. Talking about the future impacts of their novel study, Associate Prof. Masaike elaborates, “Since F1-ATPase is the world’s smallest biological rotational motor protein, it can be used as a reference to understand the mechanism of energy transduction in living organisms. This knowledge can be revolutionary in developing efficient nanomachines. Moreover, ATP synthase from Mycobacterium tuberculosis has recently been identified as a potential target for drug discovery. Therefore, to stop its rotation using inhibitors, understanding the mechanism of rotation is quite important.”
Indeed, understanding the world’s smallest biological motor may unravel mysteries of energy transduction in living organisms, and may even translate to advanced applications across disciplines!
***
Reference
DOI: https://doi.org/10.1016/j.bpj.2022.12.027
Authors: Yuh Hasimoto1,*, Mitsuhiro Sugawa2, Yoshihiro Nishiguchi3, Fumihiro Aeba4, Ayari Tagawa4, Kenta Suga4, Nobukiyo Tanaka4, Hiroshi Ueno5, Hiroki Yamashita4, Ryuichi Yokota4, Tomoko Masaike4,*, and Takayuki Nishizaka3,*
Affiliations:
1Tsukuba Research Center, Central Research Laboratory, Hamamatsu Photonics K.K.
2Graduate School of Arts & Sciences, The University of Tokyo
3Department of Physics, Faculty of Science, Gakushuin University
4Department of Applied Biological Science, Faculty of Science and Technology, Tokyo University of Science
5Department of Applied Chemistry, Graduate School of Engineering, The University of Tokyo.
Funding information
This study was supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas [“Fluctuation & Structure” of JP16H00808 and JP26103527 (to T.N.), “Cilia & Centrosomes” of JP87003306 (to T.N.)], Grant-in-Aid for Research Activity Start-up JP22870028 (to T. M.), and PRESTO JPMJPR12L8, JST (to T. M.).
Journal
Biophysical Journal
DOI
10.1016/j.bpj.2022.12.027
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Direct identification of the rotary angle of ATP cleavage in F1-ATPase from Bacillus PS3
Article Publication Date
7-Feb-2023
COI Statement
The authors declare no competing interests.




