Credit: Takenobu Group
Untwisting chains of atoms within a plastic polymer improves its ability to conduct electricity, according to a report by researchers, led by Nagoya University applied physicist Hisaaki Tanaka, in the journal Science Advances. The insight could help accelerate the development of wearable power sources for a vast number of Internet-of-things devices.
The ‘smart’ societies of the future are expected to contain a large number of electronic devices that are interconnected through the Internet: the so-called Internet-of-things. Scientists have been looking for ways to use body heat to charge some types of micro-devices and sensors. But this requires lightweight, non-toxic, wearable, and flexible thermoelectric generators.
Plastics that can conduct electricity, called conducting polymers, could fill this bill, but their thermoelectric performance needs to be improved. Their thin films have highly disordered structures, formed of crystalline and non-crystalline parts, making it notoriously difficult to understand their properties and thus find ways to optimize their performance.
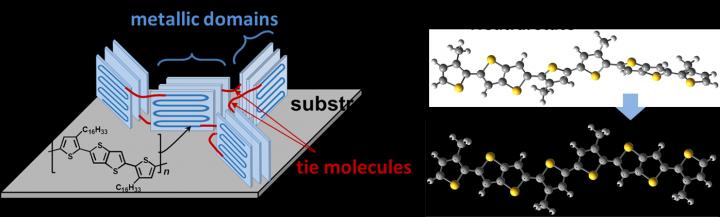
Tanaka worked with colleagues in Japan to understand the thermoelectric properties of a highly conductive thiophene-based polymer, called PBTTT. They added or ‘doped’ the polymer with a thin ion electrolyte gel, which is known to improve conductivity. The gel only infiltrates the polymer successfully when a specific electric voltage is applied.
They used a variety of measurement techniques to understand the polymer’s electronic and structural changes when doped. They found that, without the electrolyte gel, the PBTTT chain is highly twisted. Doping it with a critical amount of electrolyte untwists the chain and creates links between its crystalline parts, improving electron conductivity.
The scientists report that the formation of this interconnected conductive network is what determines the polymer’s maximum thermoelectric performance, which they were able to uniquely observe in this study.
They are now looking into ways to optimize the thermoelectric performance of thin film conducting polymers through material design and changing the fabrication conditions.
###
The article, “Thermoelectric properties of a semicrystalline polymer doped beyond the insulator-to-metal transition by electrolyte gating,” was published in the journal Science Advances on February 14, 2020 at DOI: 10.1126/sciadv.aay8065.
This work was financially supported by Grant-in-Aid for Scientific Research (JP17H01069 and 19K22127) and a Grant-in-Aid for Scientific Research on Innovative Areas (JP26102012) from the Japan Society for the Promotion of Science (JSPS) and by JST CREST (JPMJCR17I5).
For more information, contact:
Prof. Taishi Takenobu
Graduate School of Engineering, Nagoya University
Email: [email protected]
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Media Contact
Taishi Takenobu
[email protected]
Original Source
http://en.
Related Journal Article
http://dx.




