Credit: Shireen Dooling for the Biodesign Institute at ASU
Since the discovery of Alzheimer’s disease over a century ago, two hallmarks of the devastating illness have taken center stage.
The first, known as amyloid plaques, are dense accumulations of misfolded amyloid protein, occurring in the spaces between nerve cells. Most efforts to halt the advance of Alzheimer’s disease have targeted amyloid protein plaques. To date, all have met dispiriting failure.
The second classic trait has, until recently, received less scrutiny. It consists of string-like formations within the bodies of neurons, produced by another crucial protein– tau. These are known as neurofibrillary tangles.
In a new study, researchers with the ASU-Banner Neurodegenerative Disease Center at the Biodesign Institute and their colleagues investigate these tangles in the brain — pathologies not only characteristic of Alzheimer’s but other neurodegenerative conditions as well.
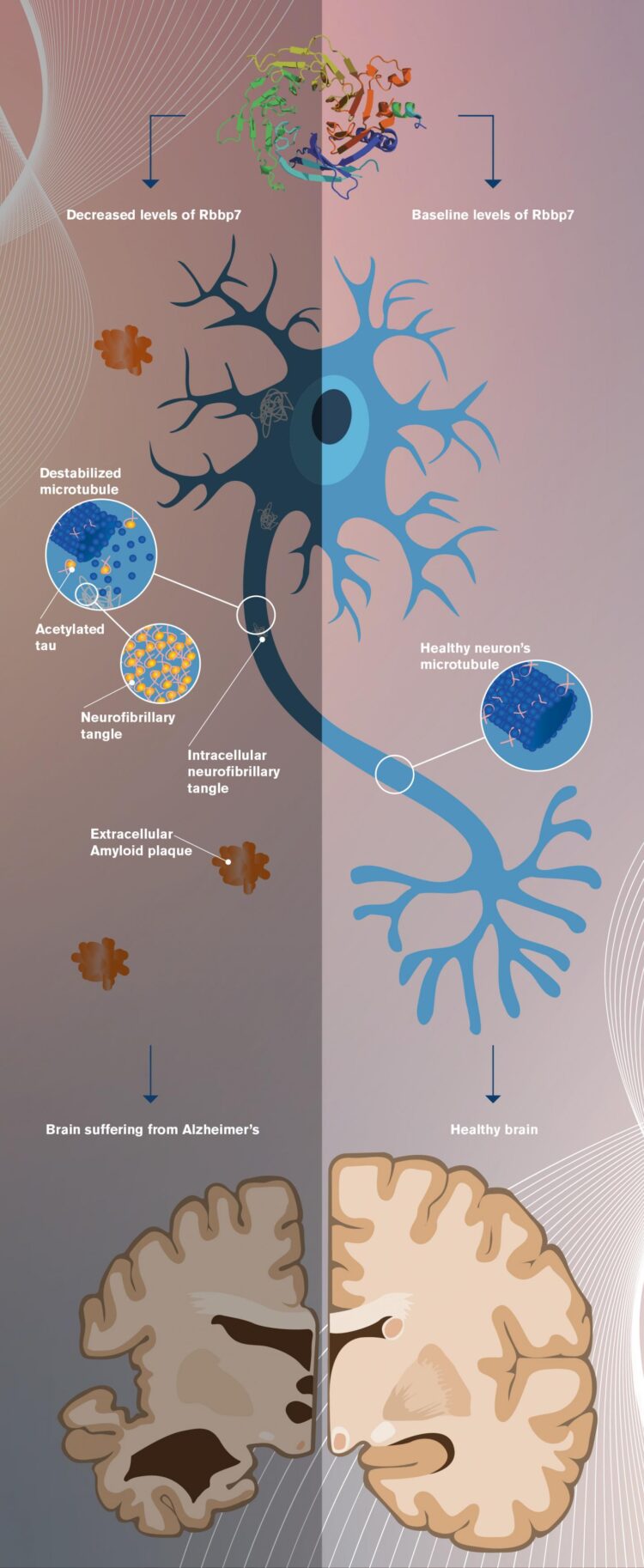
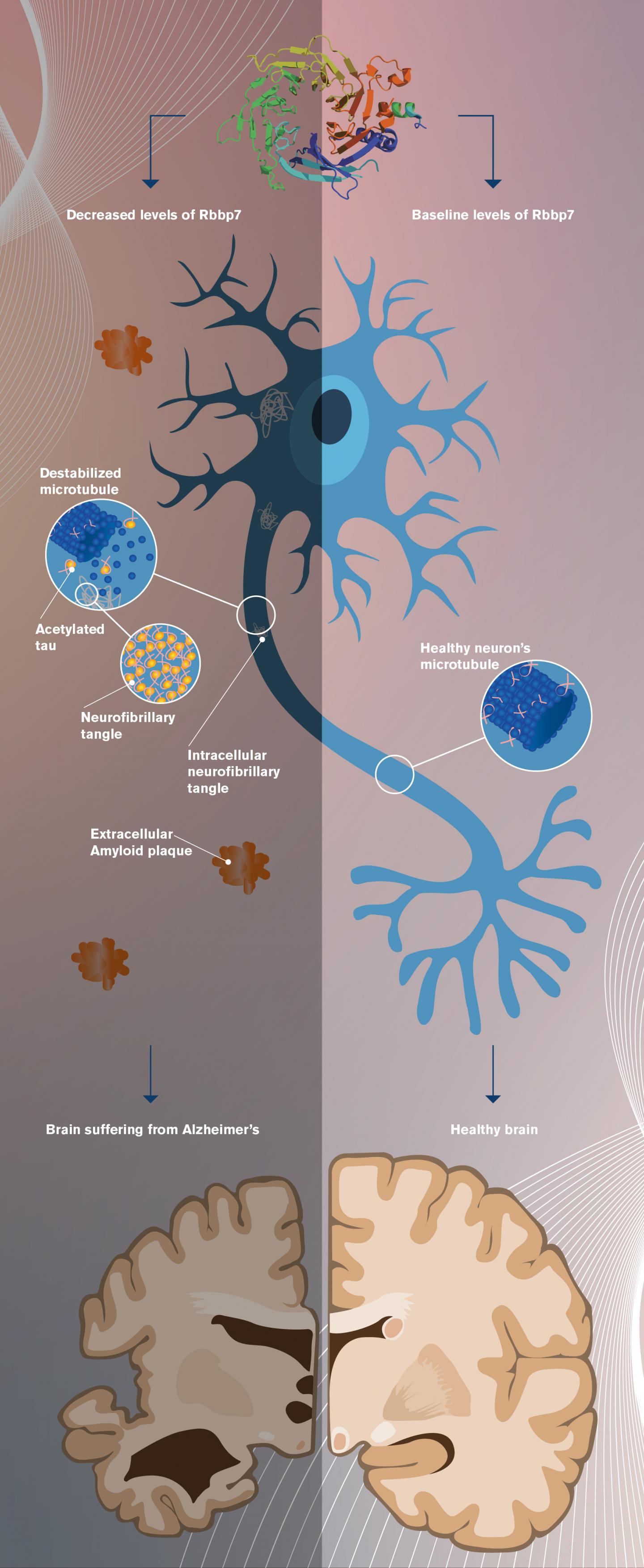
The research homes in on a particular protein known as Rbbp7, whose dysregulation appears linked to the eventual formation of tau protein tangles and the rampant cell death associated with Alzheimer’s and other neurodegenerative diseases.
“We had a hunch that this protein was involved in Alzheimer’s disease, particularly because we know that the protein was decreased in Alzheimer’s disease post-mortem brain tissue when compared with normal brains,” says Nikhil Dave, lead author of the new study.
The research shows a correlation between decreased Rbbp7 levels and increased tangle formation and associated neuronal loss and brain weight reduction in Alzheimer’s diseased brains. Intriguingly, cell loss and tangle formation were reversed in transgenic mice whose levels of Rbbp7 were restored to baseline levels.
The findings open a new avenue of research that could aid in the development of effective treatments for Alzheimer’s disease along with a broad range of tau-related afflictions, collectively known as tauopathies, including Pick’s disease, frontotemporal dementia and traumatic brain injury.
The new study appears in the current issue of the journal Acta Neuropathologica.
Infernal assault
Alzheimer’s disease remains one of the most enigmatic illnesses known to medical science. Its clinical symptoms stealthily appear over a period of years and can be masked by the normal processes of aging. Once it has taken command of the brain however, the advance of the disease is often swift and merciless.
Patients may experience a bewildering range of symptoms including confusion, physical disorientation, delusions, forgetfulness, aggression, agitation, and progressive loss of motor control.
Researchers now know that by the time the first outward appearances of the disease become apparent, Alzheimer’s disease has been silently ravaging the brain for decades, typically leaving its calling card in the form of plaques and tangles.
Alzheimer’s disease remains the leading cause of dementia, with advancing age being the primary risk factor. The disease has been on a frightening upward trajectory, as life expectancy increases and other once-deadly illnesses have become treatable, if not curable. Currently, 5.8 million people in the United States alone suffer from Alzheimer’s disease, with the number expected to swell to 14 million by 2060, according to the Centers for Disease Research.
Many other factors apart from advancing age play a role in this complex disorder, from hereditary predisposition to vascular afflictions such as diabetes and obesity. Lifestyle choices, including diet and exercise, can also affect vulnerability. The disease typically afflicts those over 65, though early onset versions of the disorder can strike much sooner.
Genome 2.0
The new study examines another area of risk for neurodegenerative illness, one bearing on an individual’s genes and how they are expressed. Although the three billion letter DNA code making up an individual’s genome remains fixed throughout life, researchers now know that chemical messengers of great variety and complexity can act on the genome, delivering instructions to the DNA and guiding its behavior.
These epigenetic changes as they are known can turn genes on and off or regulate the amount of protein these genes produce. Earlier notions in biology emphasizing a static view of genomic destinies have given way to a new picture of life in which environmental changes can profoundly affect the way our genes behave. Scientists are just beginning to learn the far-reaching influence of the epigenome on human health and disease.
The current research describes epigenetic changes that take place in the brain when the level of the protein Rbbp7 is reduced, something the researchers detected in post-mortem brain tissue from Alzheimer’s patients.
One function of Rbbp7 is to regulate gene expression. It does this by altering the interaction of DNA with proteins known as histones, which DNA wraps around like sewing thread around a spool. When the DNA thread is loosely wrapped around the histone spool, the cell machinery can read the exposed DNA message and transcribe it into mRNA, which is then translated into protein. If the DNA thread is tightly wrapped around the histone however, the DNA genes are hidden from view and transcription maybe partially or entirely blocked, thereby reducing or disabling protein expression.
The researchers observed that when Rbbp7 levels are reduced, the level of another protein known as p300 increases, causing a post-translational modification of tau protein, known as acetylation. The effect of this is to cause tau protein to detach from cell structures known as microtubules, which tau typically binds with. The detached tau is then free to accumulate within neurons, eventually forming the telltale tangles associated with Alzheimer’s disease. (See accompanying graphic.)
The acetylation of tau caused by low Rbbp7 results in increased phosphorylation of tau, further promoting tangle formation and subsequent neuronal loss in the brain.
In the new study, transgenic mice displaying tau pathology showed decreased levels of Rbbp7 and increased neuronal loss. Restoring Rbbp7 to normal levels in the mice reversed these pathologies, though the cognitive deficits remained.
Ramon Velazquez, corresponding senior author of the new study, speculates that the reason for this is that the study targeted only a small subregion of the hippocampus, while other brain areas associated with cognition were still rampant with tangle formation. “We plan to look at the global effect of overexpressing Rbbp7 in our future research to see if we can rescue learning, memory and other facets of cognition.”
Light at the end of the tunnel?
The associations outlined in the study between Rbbp7 levels and the formation of tau tangles, cell death and loss of cognitive function in the brain are compelling. The results suggest that Rbbp7 may be an attractive target for drug discovery and the development of effective therapies for Alzheimer’s disease and other tau-associated afflictions. Treatments based on studies of this kind could be ready for clinical trials within the next five years.
Nevertheless, the authors stress that other molecular players are likely involved in these complex processes. In future studies, the researchers plan to perform extensive, unbiased probing of protein interactions, transcription pathways from DNA to mRNA and the epigenetic modifications that can lead to neurodegenerative disease.
###
Media Contact
richard harth
[email protected]
Original Source
https:/
Related Journal Article
http://dx.