Lipid Metabolism’s Pivotal Role in Breast Cancer Progression and Drug Resistance: New Insights into a Metabolic Frontier
Recent advances in cancer biology have shed unprecedented light on the complex metabolic adaptations that breast cancer cells undergo to sustain their malignant phenotype. Among these, alterations in lipid metabolism stand out as critical determinants influencing tumor growth, metastasis, and therapeutic outcomes, particularly within the aggressive subtype known as triple-negative breast cancer (TNBC). Understanding the nuanced reprogramming of lipid pathways offers promising avenues to disrupt cancer progression and counteract treatment resistance.
Central to these metabolic shifts is the enhanced uptake and de novo synthesis of fatty acids, which not only fulfill the bioenergetic demands of rapidly proliferating cancer cells but also supply essential components for membrane biogenesis and signaling cascades. Enzymes such as fatty acid synthase (FASN) and transport proteins including CD36 and fatty acid-binding protein 4 (FABP4) are markedly upregulated in breast tumor cells. This overexpression drives lipid accumulation, facilitating membrane remodeling that supports cellular motility and invasiveness—a hallmark of metastatic competence.
Cholesterol metabolism also emerges as a vital axis in breast cancer pathobiology. Elevated cholesterol biosynthesis alongside increased levels of 27-hydroxycholesterol (27HC), a bioactive cholesterol metabolite, has been implicated in accelerating tumor progression. 27HC functions dually as an agonist for estrogen receptors and modulator of immune cell behavior within the tumor microenvironment. Proteins integral to cholesterol homeostasis, including SREBP2, NSDHL, and STARD4, are often dysregulated, reinforcing malignant phenotypes and enabling immune evasion by remodeling immune checkpoints and inflammatory responses.
The sphingolipid metabolic network reveals an intriguing paradox in breast cancer biology. Ceramide accumulation is recognized for its tumor-suppressive capacity, promoting apoptosis and enhancing sensitivity to chemotherapeutic agents. Conversely, glycosylated sphingolipid derivatives such as Globo-H ceramide and GD2 are associated with tumorigenesis, angiogenesis, and the maintenance of cancer stem-like cells. This dichotomy underscores the complexity of lipid-mediated signaling pathways and their divergent roles in cancer cell fate determination.
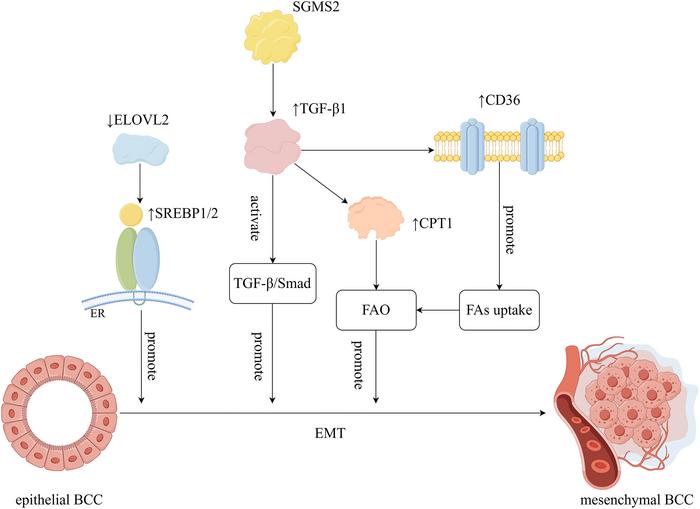
A particularly nefarious consequence of lipid metabolic reprogramming lies in its facilitation of epithelial-to-mesenchymal transition (EMT). EMT endows cancer cells with increased migratory capabilities and resistance to apoptosis, an effect mediated by molecular regulators such as the fatty acid elongase ELOVL2, sphingomyelin synthase 2 (SGMS2), and chemokine CXCL8. These factors orchestrate intricate signaling networks involving TGF-β, PI3K/AKT, and sterol regulatory element-binding proteins (SREBPs), effectively rewiring gene expression programs to favor a mesenchymal, invasive phenotype.
Moreover, the tumor immune microenvironment (TIME) is not a passive bystander but dynamically adapts to lipid-driven signals. Immunosuppressive M2 macrophages and cancer-associated fibroblasts (CAFs) reprogram their lipid metabolism, reshaping cytokine profiles and extracellular matrix components to support tumor progression. Simultaneously, cytotoxic CD8+ T cells experience lipid-mediated functional impairment, weakening anti-tumor immunity and contributing to immune evasion mechanisms.
Resistance to conventional breast cancer therapies—including chemotherapy, endocrine treatments, HER2-targeted agents, and immune checkpoint blockade—is deeply intertwined with lipid metabolic rewiring. Tumor cells amplify expression of lipid transporters like CD36, lipid synthesis enzymes such as FASN, and mitochondrial fatty acid oxidation components including CPT1, thereby circumventing apoptotic triggers and diminishing intracellular drug accumulation. G protein-coupled receptor 120 (GPR120) further mediates pro-survival lipid signaling contributing to the maintenance of cancer stemness and therapy resistance.
Beyond intrinsic cancer cell alterations, cross-talk between lipid metabolism and oncogenic signaling pathways reveals additional complexity. Activation of SREBP1/2 transcription factors regulates genes involved in lipid biosynthesis and uptake, coalescing with pathways modulated by TGF-β and PI3K/AKT to promote malignant behaviors. Downregulation of enzymes like ELOVL2 disrupts lipid homeostasis, potentiating EMT and metastatic dissemination, highlighting the interplay between metabolic enzymes and cellular phenotypic plasticity.
The role of cholesterol metabolites extends past tumor intrinsic functions, as 27HC modulates local immunosuppression by influencing tumor-infiltrating lymphocytes and facilitating escape from immune surveillance. This duality creates therapeutic challenges, particularly in hormone receptor-positive breast cancers where estrogen signaling intersects with lipid metabolic pathways, necessitating refined strategies to target this metabolic-immune axis.
Emerging therapeutic interventions aimed at targeting lipid metabolism show potential in overcoming drug resistance. Pharmacological inhibitors of FASN, blockers of CD36-mediated fatty acid uptake, and modulators of sphingolipid pathways are under investigation for their capacity to sensitize tumors to existing treatments. Combinatorial approaches targeting lipid metabolism alongside conventional or immune-based therapies may enable more durable responses.
Encapsulating these insights, lipid metabolic reprogramming represents a fundamental axis driving breast cancer progression, metastatic competence, immune evasion, and therapeutic resistance. Deciphering the molecular underpinnings of this metabolic landscape opens promising avenues for novel biomarker identification and the development of metabolism-targeted therapies that could revolutionize breast cancer management.
As research delves deeper into the metabolic vulnerabilities of breast cancer, especially aggressive subtypes like TNBC, the integration of lipidomics with genomic and proteomic data stands to refine precision oncology approaches. Such multidisciplinary efforts are essential to translating mechanistic discoveries into clinical gains, ultimately improving patient survival and quality of life.
Subject of Research: Lipid metabolism in breast cancer progression and therapy resistance, focusing on metabolic reprogramming mechanisms in triple-negative breast cancer.
Article Title: Lipid metabolism involved in progression and drug resistance of breast cancer.
Web References: http://dx.doi.org/10.1016/j.gendis.2024.101376
References: Wenxiang Fu, Aijun Sun, Huijuan Dai, Lipid metabolism involved in progression and drug resistance of breast cancer, Genes & Diseases, Volume 12, Issue 4, 2025, 101376
Image Credits: Genes & Diseases
Keywords: Breast cancer, lipid metabolism, fatty acids, cholesterol, sphingolipids, epithelial-mesenchymal transition, drug resistance, tumor microenvironment, triple-negative breast cancer, FASN, CD36, 27-hydroxycholesterol
Tags: cancer cell bioenergetics and lipidscholesterol metabolism in breast cancerenzymes in lipid metabolism cancerfatty acid synthesis in cancer cellslipid accumulation and cancer invasivenesslipid metabolism in breast cancermembrane biogenesis in breast tumorsmetabolic adaptations in tumor growthmetabolic reprogramming in cancerrole of fatty acid transport proteins in cancertherapeutic targets in lipid metabolismtriple-negative breast cancer therapy resistance