Scientists unlock the secret about how abnormality in the production of the mysterious “tau” protein leads to neurodegenerative diseases.
Credit: Shinsuke Ishigaki
Frontotemporal lobar degeneration (FTLD) is a type of dementia that appears earlier in life than Alzheimer’s disease (AD). Both FTLD and AD, along with several other neurodegenerative diseases, are marked by the appearance and clustering of the protein “tau” in nerve cells. However, there is much left to be explored about this mechanism.
Now, a team of researchers from various collaborating universities and hospitals in Japan has uncovered crucial molecular details regarding tau’s activity, promising to revolutionize the therapy of tau-induced neurodegenerative diseases. Their findings were recently published in the journal BRAIN.
Tau-induced neurodegenerative diseases include not only FTLD and AD, but also an array of conditions like amyotrophic lateral sclerosis (ALS), progressive supranuclear palsy (PSP), and corticobasal degeneration (CBD). Many people from various age groups are affected by the tau-induced diseases, but the effective therapeutic strategy against tau aggregation is yet to be available. One reason behind this gap is that, despite a lot of effort and resource invested, the exact mechanism of the action of tau inside the cell is still unclear. Knowing this will help us pin down an appropriate treatment strategy.
The aforementioned research team, led by Dr Shinsuke Ishigaki of Nagoya University Graduate School of Medicine, has now discovered new layers of complexities hidden in the cellular activities of tau. The researchers report a novel role of tau that is specific for FTLD spectrum diseases, and as per their findings published in BRAIN, these finer mechanisms specifically contribute to the development of conditions such as FTLD, ALS, PSP, and CBD, but not in AD and Pick’s diseases.
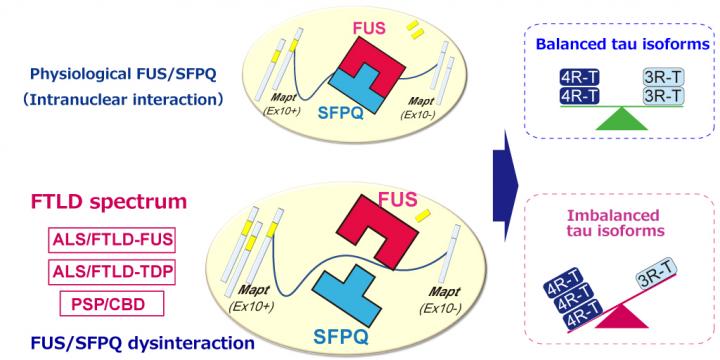
However, while this research puts tau in the spotlight, it all started with another related protein. Dr Ishigaki explains the reasons for arriving at their study question: “Earlier, while studying FTLD mouse models, we found two interacting proteins, fused in sarcoma (FUS) and splicing factor, proline- and glutamine-rich (SFPQ), were important for the generation of functional tau. The interaction of FUS and SFPQ inside the nucleus is disrupted due to mutations in FUS results in neurodegeneration by the accumulation of a dysfunctional variant ‘4-repeat tau’, causing FTLD in mouse.”
So how did the researchers link their findings of mouse model to the tau-induced pathogenesis in humans? They studied the interaction of SFPQ and FUS in brain autopsy samples of 142 deceased individuals with various neurodegenerative diseases like FUS-related ALS/FTLD, TDP-43-related ALS/FTLD, PSP, AD, or Pick’s disease, with the latter disease used as a control to compare the results.
Using their findings, the researchers have proposed a unique model of “imbalanced accumulation of tau” in cells. As per this new model, FUS and SFPQ regulate the processing of MAPT, the gene that “codes” for tau, specifically by removing a genetic region called exon 10. In normal conditions, the balance in the ratio of variants “4-repeat tau” and “3-repeat tau” is maintained by MAPT. In disease conditions, the processing of MAPT is hampered, leading to an unchecked increase in the amount of 4-repeat tau. Interestingly, an increased level of 4-repeat tau causes FTLD spectrum diseases, but not AD or Pick’s disease, in humans.
“Now that we know how tau specifically causes FTLD spectrum diseases, we can design a treatment strategy for these diseases that could ‘target’ the factors involved in the process, like ‘4-repeat tau’ or FUS/SFPQ proteins,” concludes Dr Ishigaki, talking about the significance of their discovery.
###
The paper, “Aberrant interaction between FUS and SFPQ in neurons in a wide range of FTLD spectrum diseases,” was published in the journal BRAIN on August 8, 2020 at DOI: 10.1093/brain/awaa196.
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Media Contact
Shinsuke Ishigaki
[email protected]
Original Source
http://en.
Related Journal Article
http://dx.




