A new rubber band stretches, but then snaps back into its original shape and size. Stretched again, it does the same. But what if the rubber band was made of a material that remembered how it had been stretched? Just as our bones strengthen in response to impact, medical implants or prosthetics composed of such a material could adjust to environmental pressures such as those encountered in strenuous exercise.
A research team at the University of Chicago is now exploring the properties of a material found in cells which allows cells to remember and respond to environmental pressure. In a paper published on May 14, 2021 in Soft Matter, they teased out secrets for how it works–and how it could someday form the basis for making useful materials.
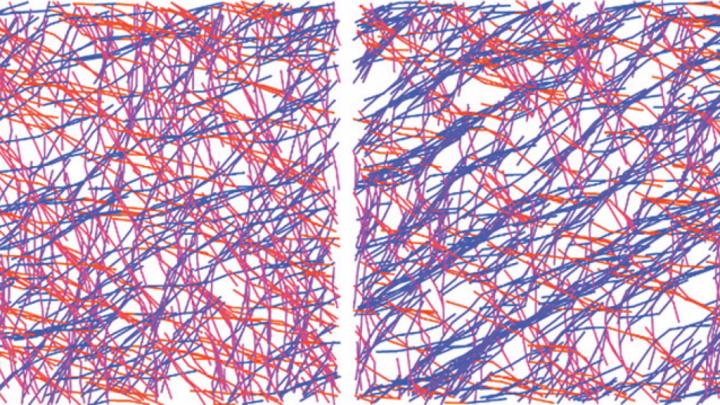
Protein strands, called actin filaments, act as bones within a cell, and a separate family of proteins called cross-linkers hold these bones together into a cellular skeleton. The study found that an optimal concentration of cross-linkers, which bind and unbind to permit the actin to rearrange under pressure, allow this skeletal scaffolding to remember and respond to past experience. This material memory is called hysteresis.
“Our findings show that the properties of actin networks can be changed by how filaments are aligned,” said Danielle Scheff, a graduate student in the Department of Physics who conducted the research in the lab of Margaret Gardel, Horace B. Horton Professor of Physics and Molecular Engineering, the James Franck Institute, and the Institute of Biophysical Dynamics. “The material adapts to stress by becoming stronger.”
To understand how the composition of this cellular scaffolding determines its hysteresis, Scheff mixed up a buffer containing actin, isolated from rabbit muscle, and cross-linkers, isolated from bacteria. She then applied pressure to the solution, using an instrument called a rheometer. If stretched in one direction, the cross-linkers allowed the actin filaments to rearrange, strengthening against subsequent pressure in the same direction.
To see how hysteresis depended on the solution’s consistency, she mixed different concentrations of cross-linkers into the buffer.
Surprisingly, these experiments indicated that hysteresis was most pronounced at an optimal cross-linker concentration; solutions exhibited increased hysteresis as she added more cross-linkers, but past this optimal point, the effect again became less pronounced.
“I remember being in lab the first time I plotted that relationship and thinking something must be wrong, running down to the rheometer to do more experiments to double-check,” Scheff said.
To better understand the structural changes, Steven Redford, a graduate student in Biophysical Sciences in the labs of Gardel and Aaron Dinner, Professor of Chemistry, the James Franck Institute, and the Institute for Biophysical Dynamics, created a computational simulation of the protein mixture Scheff produced in the lab. In this computational rendition, Redford wielded a more systematic control over variables than possible in the lab. By varying the stability of bonds between actin and its cross-linkers, Redford showed that unbinding allows actin filaments to rearrange under pressure, aligning with the applied strain, while binding stabilizes the new alignment, providing the tissue a ‘memory’ of this pressure. Together, these simulations demonstrated that impermanent connections between the proteins enable hysteresis.
“People think of cells as very complicated, with a lot of chemical feedback. But this is a stripped-down system where you can really understand what is possible,” said Gardel.
The team expects these findings, established in a material isolated from biological systems, to generalize to other materials. For example, using impermanent cross-linkers to bind polymer filaments could allow them to rearrange as actin filaments do, and thus produce synthetic materials capable of hysteresis.
“If you understand how natural materials adapt, you can carry it over to synthetic materials,” said Dinner.
###
The collaboration between the Gardel and Dinner labs reflects broader efforts to understand adaptive properties of materials at UChicago’s Materials Research Science and Engineering Center and the James Franck Institute.
Citation: Scheff DR, et al., (2021) Actin filament alignment causes mechanical hysteresis in cross-linked networks. Soft Matter. DOI: 10.1039/d1sm00412c
Media Contact
Cynthia Medina
[email protected]
Original Source
https:/
Related Journal Article
http://dx.