Using waste heat helps cut cost of producing hydrogen
Credit: Illustration: NTNU
In June, the International Energy Agency confirmed what most experts already know: that the world should work harder to boost the use of pure hydrogen as an emissions-free energy source.
One of the challenges of creating hydrogen, however, is that it takes energy–lots of energy. The IEA says that producing all of today’s hydrogen just using electricity would require 3600 TWh, which is more than is generated annually by the European Union.
But what if you could use an existing source of wasted energy to help with hydrogen production? A new approach developed by researchers at the Norwegian University of Science and Technology does exactly this — by using waste heat from other industrial processes.
“We’ve found a way of using heat that otherwise isn’t worth much,” said Kjersti Wergeland Krakhella, the first author of an article about the process published in the academic journal MDPI Energies. “It’s low-grade, low-temperature heat — but it can be used to make hydrogen.
One-seventh of Norway’s electricity production
Waste heat is exactly what it sounds like — heat produced as a byproduct of an industrial process. Anything from an industrial boiler to a waste-to-energy plant produces waste heat.
More times than not, this excess heat has to be released to the environment. Energy experts say that the waste heat from Norway’s businesses and industries is the equivalent of 20 TWh of energy.
To put this in perspective, Norway’s entire hydropower system produces 140 TWh of electricity a year. That means there’s a lot of waste heat out there that could potentially be put to work.
Membranes and salts
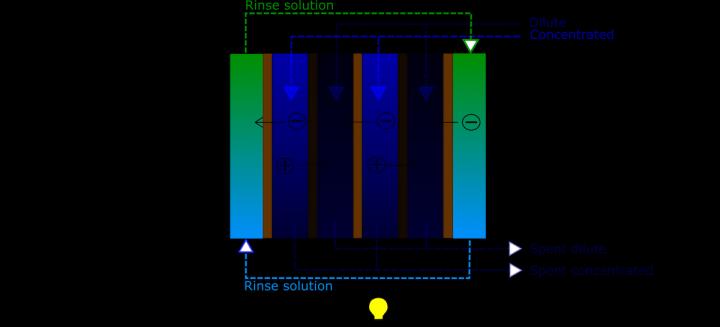
The researchers used a technique called reverse electrodialysis (RED), which relies on salt solutions and two varieties of ion exchange membranes.
To understand what the researchers actually did, you first have to understand how the RED technique works.
In RED, one membrane, called the anion exchange membrane, or AEM, allows negatively charged electrons (anions) to move through the membrane, while a second membrane, called the cation exchange membrane, or CEM, allows positively charged electrons (cations) to flow through the membrane.
The membranes separate a dilute salt solution from a concentrated salt solution. The ions migrate from the concentrated to the dilute solution, and because the two different types of membranes are alternated, they force the anions and cations to migrate in opposite directions.
When these alternating columns are sandwiched between two electrodes the stack can generate enough energy to split water into hydrogen (on the cathode side) and oxygen (on the anode side).
This approach was developed in the 1950s and first used saltwater and river water.
What Krakhella and her colleagues did, however, was to use a different kind of salt called potassium nitrate. The use of this kind of salt enabled them to use waste heat as part of the process.
Reusing the salts using waste heat
If you run the RED stacks described above, at some point the concentrate and dilute salt solutions become more and more alike, so they have to be refreshed.
That means you need to find a way to increase the concentration of the salt in the concentrated solution and remove salt from the dilute solution. That’s where the waste heat comes in.
The researchers tested two systems.
The first was where waste heat was used to evaporate water from the concentrated solution to make it more concentrated.
The second system used waste heat to cause salt to precipitate out of the diluted solution (so it will be less salty).
“If you find a way to remove the water or remove the salt, you have done the job,” Krakhella said.
Both had benefits
When the researchers looked at their results, they saw that using existing membrane technology and waste heat to evaporate water from their system produced more hydrogen per membrane area than the precipitation approach.
The production of hydrogen was four times higher for the evaporation system operated at 25 C and two times higher for a system operated at 40 C compared to their precipitation system.
That made it a better candidate from a cost perspective.
However, the precipitation process was better in terms of energy demand, the researchers found. For example, the energy needed to produce a cubic meter of hydrogen using the precipitation process was just 8.2 kWh, compared to 55 kWh for the evaporation process.
New system with many possibilities
While Krakhella’s work proves the concept will work, she’s mostly worked with a lab bench model and lots of computer calculations. There’s still lots of work to be done, especially with respect to the kind of salt used in the process.
The researchers chose potassium nitrate for their salt system, but other salts could also work, she said.
“It’s a completely new system,” she said. “We’ll need to do more testing with other salts at other concentrations.”
Membrane prices are limiting factor
Another issue that continues to limit hydrogen production is that the membranes themselves remain extremely costly.
Krakhella hopes that as societies look to move away from fossil fuels, increased demand will drive the price of membranes down, as well as improving the characteristics of the membranes themselves.
“The membranes are the most expensive part of our system,” Krakhella said. “But everyone knows we need to do something about the environment, and the price is potentially much higher for society if we don’t develop pollution-free energy.”
###
Reference: Heat to H2: Using Waste Heat for Hydrogen Production through Reverse Electrodialysis. Kjersti Wergeland Krakhella, Robert Bock, Odne Stokke Burheim and Frode Seland and Kristian Etienne Einarsrud. Energies 2019, 12, 3428; doi:10.3390/en12183428
Media Contact
Odne Stokke Burheim
[email protected]
47-917-07856
Original Source
https:/
Related Journal Article
http://dx.




