Did you know rivers carry about 40 trillion metric tons of river water into the ocean every year? This meeting point, known as the estuary, holds great potential for electricity generation. Mixing the two types of water — seawater and river water containing different salt concentrations — releases a substantial amount of Gibbs free energy, which can be converted to electricity using semipermeable membranes. However, the performance of membranes has limited the economic viability of membrane-based approaches, leaving the vast potential of this naturally abundant energy source largely untapped.
Credit: Research
Did you know rivers carry about 40 trillion metric tons of river water into the ocean every year? This meeting point, known as the estuary, holds great potential for electricity generation. Mixing the two types of water — seawater and river water containing different salt concentrations — releases a substantial amount of Gibbs free energy, which can be converted to electricity using semipermeable membranes. However, the performance of membranes has limited the economic viability of membrane-based approaches, leaving the vast potential of this naturally abundant energy source largely untapped.
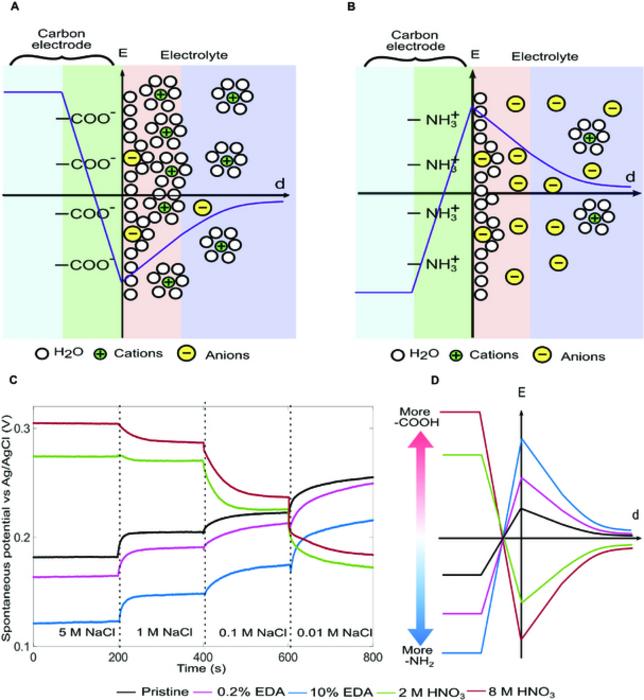
To overcome the challenges associated with the membranes, researchers have developed membrane-free technologies. Instead of using membranes to create a boundary between saltwater and freshwater for mixing, they mix directly in the nanopores of capacitive electrodes. In particular, mixing induces structural changes of the electric double layers (EDLs) that spontaneously form at a solid-electrolyte interface — the surface of the pores in this case. When freshwater is introduced to a pore filled with seawater, the salt concentration in the pore will reduce, making the EDLs expand – much the same way as moving the two plates of a parallel-plate capacitor away from each other. As a result, the Gibbs free energy driving the expansion is converted to electrical energy stored in the EDLs. However, the low surface charge of existing electrode systems has been a major challenge for this concept to be a promising contender as an alternative to membrane-based approaches – the benefits of getting rid of membranes remain hypothetical.
Researchers at Texas A&M University-Corpus Christi, the University of Hawaii, and the Beijing Institute of Nanoenergy and Nanosystems have recently developed a new electrode system that demonstrated a much higher level of surface charge when submerged in aqueous electrolytic solutions (DOI: 10.34133/research.0173). By treating the surface of a porous, activated carbon material differently, the researchers were able to modify the surface molecular structures by attaching functional groups that induce opposite surface charges to the surface. In particular, when immersed in a sodium chloride solution, the surface groups on one electrode each loses one hydrogen atom while those on the other electrode each gains one hydrogen atom, creating oppositely charged electrodes. The opposite charges increase the electrostatic energy stored in the EDLs, allowing more Gibbs free energy to be converted to electricity. Under normal seawater and freshwater condition, the new electrode system could triple the areal power density of existing capacitive systems. The volumetric power density of the prototype device is on par with or even surpasses, that of membrane-based technologies. Amazingly, the system can retain 90% of its capacity after more than 50 thousand charging/discharging cycles, making it much more economically competitive. The researchers are confident that their approach has significant room for further development. This breakthrough opens up exciting possibilities for practical, economically viable solutions to harness this abundant source of renewable energy.
Journal
Research
DOI
10.34133/research.0173
Method of Research
Experimental study
Subject of Research
Not applicable
Article Title
Tuning Surface Molecular Design of Porous Carbon for Blue Energy Harvesting
Article Publication Date
19-Jun-2023
COI Statement
T.M. and J.Y. conceived the idea. T.M. and J.Y. designed all experiments. J.Y. fabricated the capacitors. J.Y. and T.M. performed measurements and data analysis. T.M., J.Y., and Z.-L.W. wrote the manuscript.



