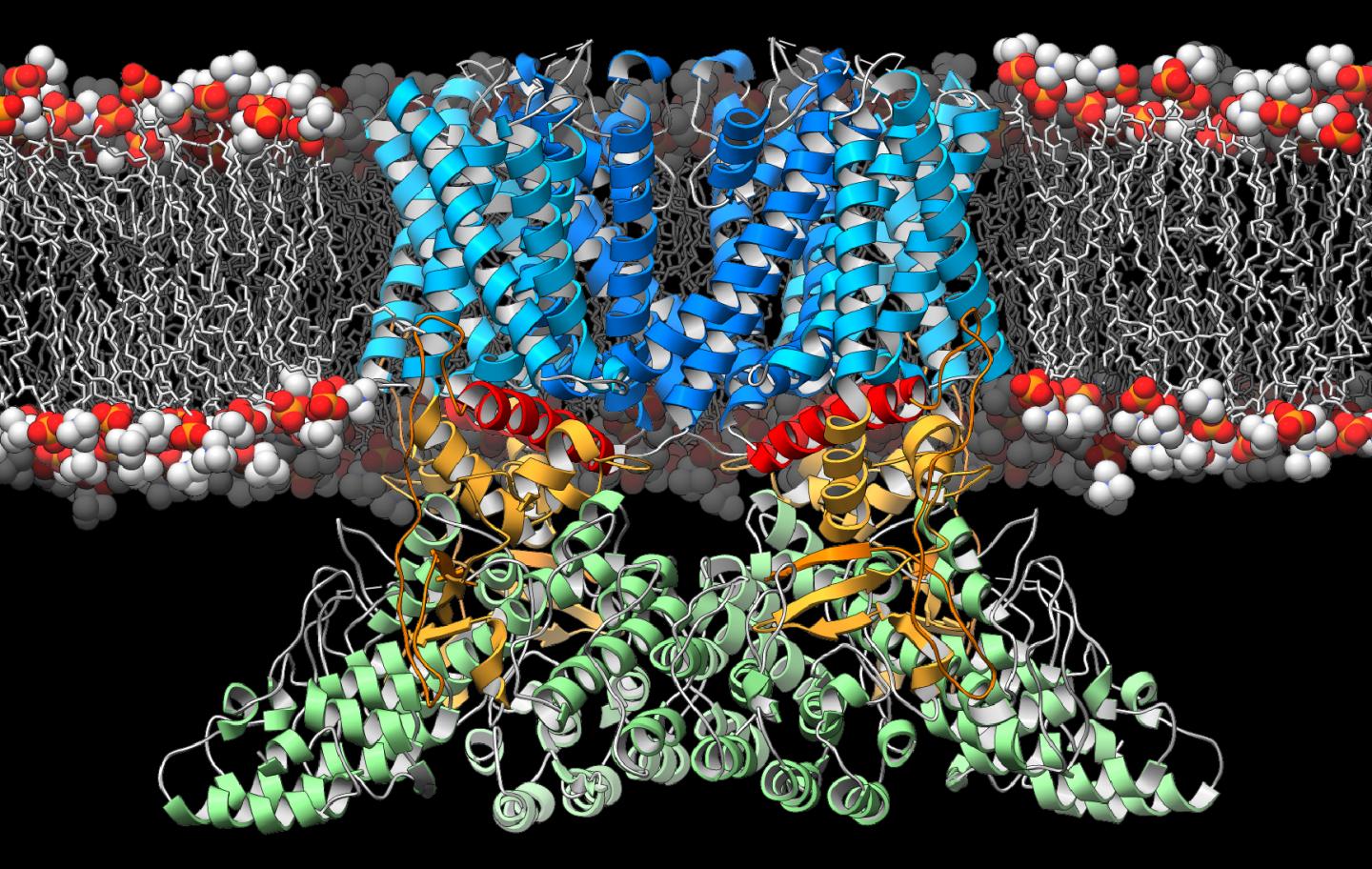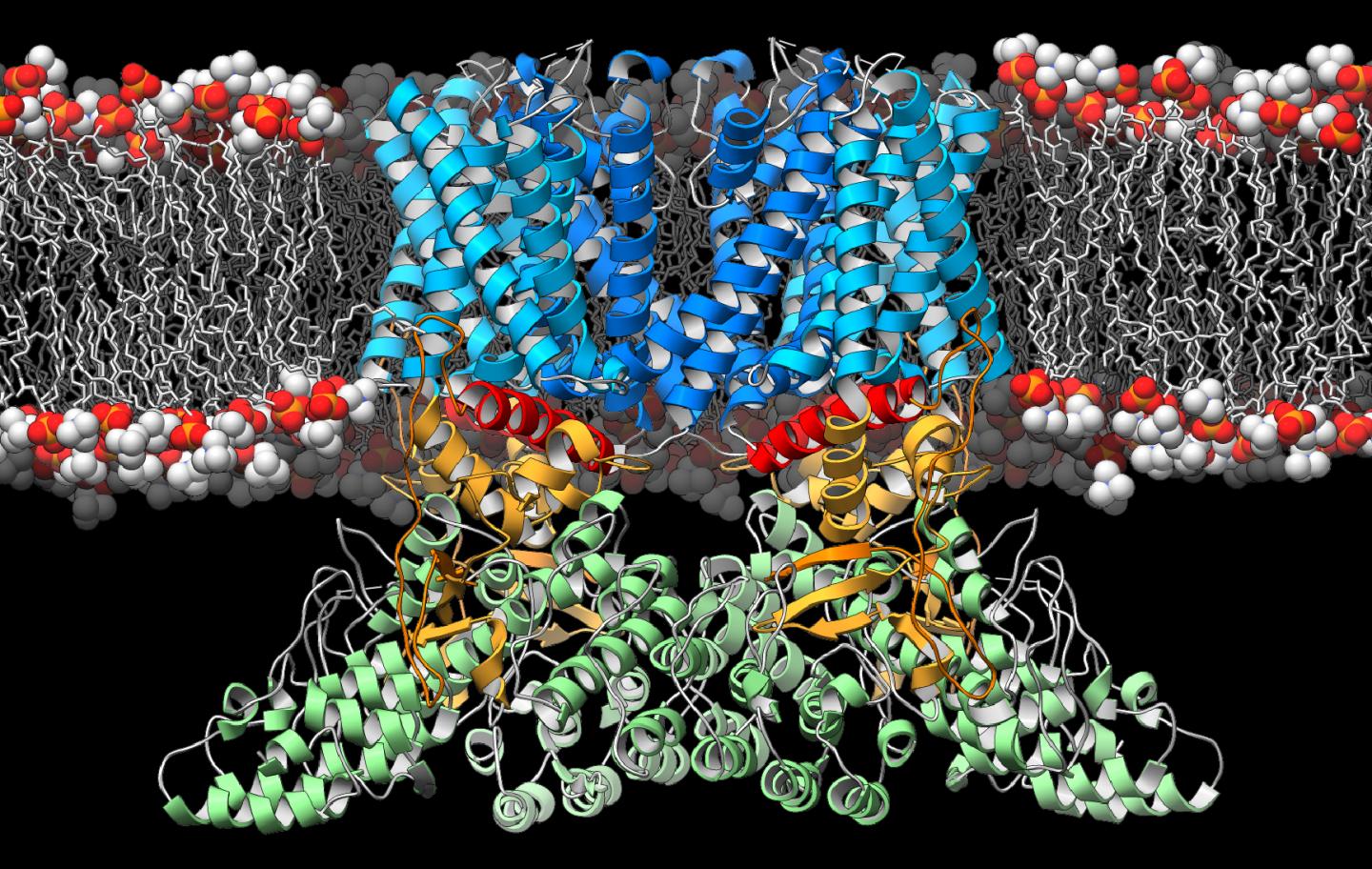
IMAGE: A new study from The Scripps Research Institute and Duke University Medical Center reveals the three-dimensional structure of a crucial ion channel, shedding light on its role in the immune…
Credit: Photo courtesy of The Scripps Research Institute.
LA JOLLA, CA – Jan. 18, 2016 – Many cells have microscopic gates, called ion channels, which open to allow the flow of ions across the cell membrane. Thanks to these gates, cells can detect stimuli such as heat, pain, pressure and even spicy food.
In a new study, researchers from The Scripps Research Institute (TSRI) and the Duke University Medical Center reveal the three-dimensional structure of a crucial ion channel. Their findings depict this channel in more detail than ever before, shedding light on the channel's possible role in immune functions such as detecting infection and inflammation.
"Our ability to perceive our environment — which includes sensing temperature and pain — is heavily reliant on these channels. Understanding their 3D structure paves the way for the development of a wide variety of new therapies," said TSRI biologist Gabe Lander, who was co-senior of author of the study with biochemist Seok-Yong Lee of the Duke University Medical Center.
The new study was published Jan. 18, 2016, in the journal Nature Structural and Molecular Biology.
An Important Sensor
Lander and his colleagues focused on an ion channel called the transient receptor potential vanilloid-2 (TRPV2), which resides within the membranes of cells throughout the body. Previous research had suggested TRPV2 was involved in sensing physical stresses, such as changes in pressure and temperature, as well as in detecting immune challenges and activating the immune system's T cells.
In the new study, the researchers used an imaging technique called cryo-electron microscopy, in which a sample is pelted with high-energy electrons. Through the use of new sample preparation techniques, computer programs and a new generation of cameras, researchers at TSRI have improved the potential resolution of cryo-electron microscopy images to the point that TRPV2 could be imaged with near-atomic precision.
"The fact that the field of cryo-electron microscopy has advanced to where we can now solve the structures of these small membrane-embedded complexes to such high resolution is exciting," said TSRI Research Associate Mark Herzik Jr., who was co-first author of the study with Lejla Zubcevic of Duke University. "The methodological insights from this study will help advance other projects in the lab."
When the researchers compared the structure of TRPV2 with TRPV1, a genetically similar ion channel found only in the nervous system, they noticed some significant differences. TRPV2's architectural components near the central gate and the peripheral domains were in a previously unobserved configuration. Together, this led the authors to propose that this configuration represents a 'desensitized' state, providing a new molecular snapshot of these ion channels at work.
"The TRVP2 ion channel is likely a global internal sensor–playing an important role in our immune response," said Lander.
Lander said the next step is to find the structures of TRPV2 at different stages of opening and closing its gate. With the entire cycle imaged, researchers will have a better idea of how the ion channel works and how it might be manipulated therapeutically to treat autoimmune diseases.
###
The other co-authors on the paper, "Cryo-electron microscopy structure of the TRPV2 ion channel," were Ben Chung and Zhirui Liu of the Duke University Medical Center.
This study was supported by the Duke University Medical Center, the National Institutes of Health (grants R01GM100894, DP2OD008380 and DP2EB020402), the Searle Scholars Program and The Pew Charitable Trusts.
Media Contact
Madeline McCurry-Schmidt
[email protected]
858-784-9254
@scrippsresearch
http://www.scripps.edu