Ovarian cancer remains one of the most challenging gynecological malignancies, notorious for its late-stage diagnosis and poor survival rates. Despite ongoing advancements in oncological therapies, the urgent pursuit for innovative treatment strategies continues. Recent studies have shed light on the role of biomarkers in not just diagnosing, but also predicting, treatment responses in ovarian cancer patients. A prominent focus of this search has been the investigation of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitors, which have emerged as promising therapeutic agents in the battle against this formidable disease.
The current research highlights the significance of TRIM4, an E3 ubiquitin ligase, in modulating ovarian cancer responses to CDK4/6 inhibitors. TRIM4 plays a crucial role in cellular regulation through its involvement in the ubiquitin-proteasome system, a key pathway for protein degradation. By affecting the stability of other proteins, TRIM4 influences various cellular processes, including cell cycle progression and apoptosis, both of which are vital for cancer development and therapy resistance.
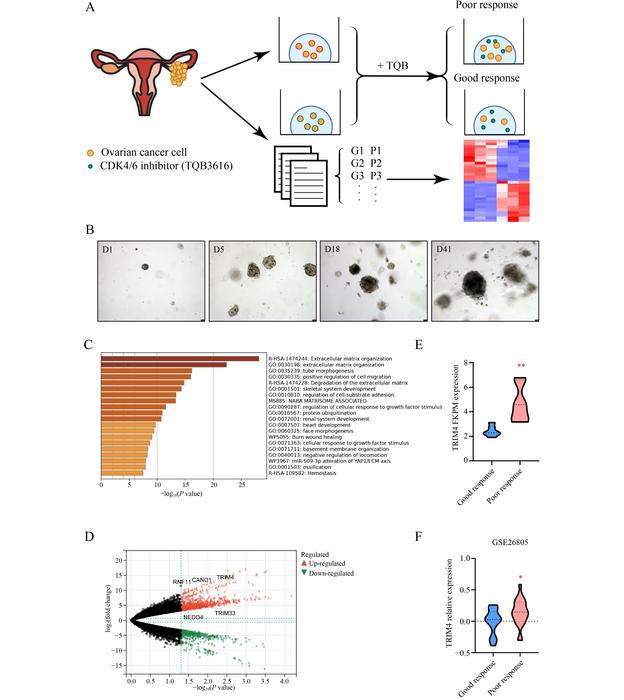
In unveiling the intricate relationship between TRIM4 and CDK4/6 inhibition, researchers established patient-derived organoid models of ovarian cancer. These organoids provide a more accurate representation of tumor behavior compared to traditional cell lines, allowing for detailed exploration of therapeutic responses. The study focused particularly on TQB3616, a novel CDK4/6 inhibitor, which was subjected to rigorous testing to assess its efficacy in inhibiting tumor growth.
RNA sequencing analyses offered compelling insights into the genetic underpinnings of resistance mechanisms in tumoral cells. The investigation revealed an elevated expression of TRIM4 in organoids resistant to TQB3616, suggesting that TRIM4 may serve as a predictive marker for drug response. This elevation in TRIM4 levels was corroborated using various clinical samples, establishing a strong correlation between TRIM4 expression and ovarian cancer responses to CDK4/6 inhibition.
The mechanism by which TRIM4 facilitates resistance was explored in detail. It was discovered that TRIM4 exerts its influence by interacting with heterogeneous nuclear ribonucleoprotein D-like (hnRNPDL), a splicing factor that plays a vital role in the regulation of CDKN2C, a key tumor suppressor gene. The study illustrated how TRIM4 mediates the ubiquitination and subsequent degradation of hnRNPDL, leading to decreased levels of this protein within the cell.
The implications of reduced hnRNPDL levels were profound. HnRNPDL is critically involved in the splicing of CDKN2C mRNA, and its degradation resulted in enhanced expression of CDKN2C. This elevation in CDKN2C, in turn, is thought to affect the sensitivity of ovarian cancer cells to CDK4/6 inhibitors, ultimately contributing to the identified resistance. Such insights reinforce the importance of not only understanding the individual roles of these proteins but also their interactions within the broader context of the tumor microenvironment.
To further substantiate these findings, researchers designed experiments to downregulate TRIM4 expression. This was achieved via specific small interfering RNA (siRNA) targeting of TRIM4, with the participants then subjected to TQB3616 treatment. The results were striking; reduced TRIM4 led to heightened sensitivity of ovarian cancer cells to the CDK4/6 inhibitor, both in vitro and in vivo.
Through methods such as cell cycle analysis and apoptosis assays, the study quantified the impact of TRIM4 modulation on cellular responses. These experiments indicated that silencing TRIM4 could effectively potentiate the effects of TQB3616, suggesting a synergistic approach for enhancing therapeutic outcomes. Notably, the combination treatment markedly reduced tumor growth in murine models, highlighting the translational potential of these findings into future clinical applications.
The culmination of this research underscores the promising role of TRIM4 as both a biomarker for CDK4/6 inhibitor response and a potential therapeutic target in ovarian cancer. As researchers continue to unravel the complexities of tumor biology, the TRIM4-hnRNPDL-CDKN2C regulatory axis presents a compelling area for further exploration. Understanding these mechanisms will be crucial in developing personalized treatment strategies for ovarian cancer patients, providing hope for improved prognoses in a disease characterized by its lethality.
With an increasing push towards precision medicine, TRIM4’s influence may pave the way for innovative treatment modalities, potentially transforming the landscape of ovarian cancer care. The intricate dance between TRIM4 and CDK4/6 inhibitors not only exemplifies the challenges faced in targeting cancer effectively but also illustrates the critical need for ongoing research to dissect the underlying biological frameworks that govern therapeutic responses.
As we stand on the brink of new discoveries, the invaluable contributions from studies such as this illuminate pathways to better outcomes for patients. By integrating molecular biology with clinical practices, the future of ovarian cancer management could very well hinge on our ability to understand and manipulate these key regulatory elements within tumor cells.
Researchers hope that by targeting TRIM4, they can develop therapies that not only improve patient responses to existing treatments but also reduce the incidence of resistance—a critical barrier in cancer therapy that often leads to treatment failure. The integration of TRIM4-focused strategies could herald a new era of targeted therapies specifically tailored to the molecular profiles of individual patients, making strides towards more effective and personalized cancer treatment in the years to come.
In conclusion, this groundbreaking research reinforces the potential of biomarker-driven approaches in oncology, calling for further investigation into TRIM4’s multifaceted roles in cancer biology. By elucidating the connections between TRIM4, hnRNPDL, and CDKN2C, the study presents a foundational framework for the next generation of therapeutic strategies aimed at conquering ovarian cancer.
Subject of Research: People
Article Title: TRIM4 modulates the ubiquitin-mediated degradation of hnRNPDL and weakens sensitivity to CDK4/6 inhibitor in ovarian cancer
News Publication Date: 24-Feb-2025
Web References: 10.1007/s11684-024-1103-5
References: None available
Image Credits: Xiaoxia Che, Xin Guan, Yiyin Ruan, Lifei Shen, Yuhong Shen, Hua Liu, Chongying Zhu, Tianyu Zhou, Yiwei Wang, Weiwei Feng
Keywords: Ovarian cancer, TRIM4, CDK4/6 inhibitors, biomarkers, E3 ligase, hnRNPDL, CDKN2C, organoid models, therapeutic strategies, drug resistance.
Tags: biomarkers in ovarian cancercancer therapy resistanceCDK4/6 inhibitors effectivenesscellular regulation mechanismsgynecological malignancies challengeslate-stage ovarian cancer diagnosisoncological therapy advancementsovarian cancer treatment strategiespatient-derived organoid modelsprotein degradation pathwaystreatment response predictionTRIM4 E3 ubiquitin ligase