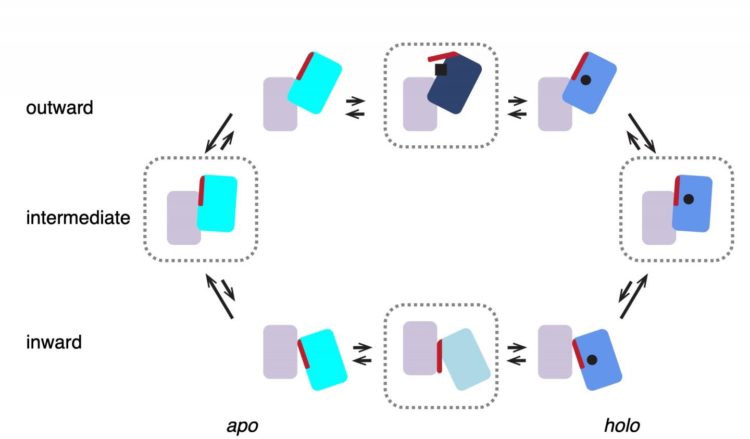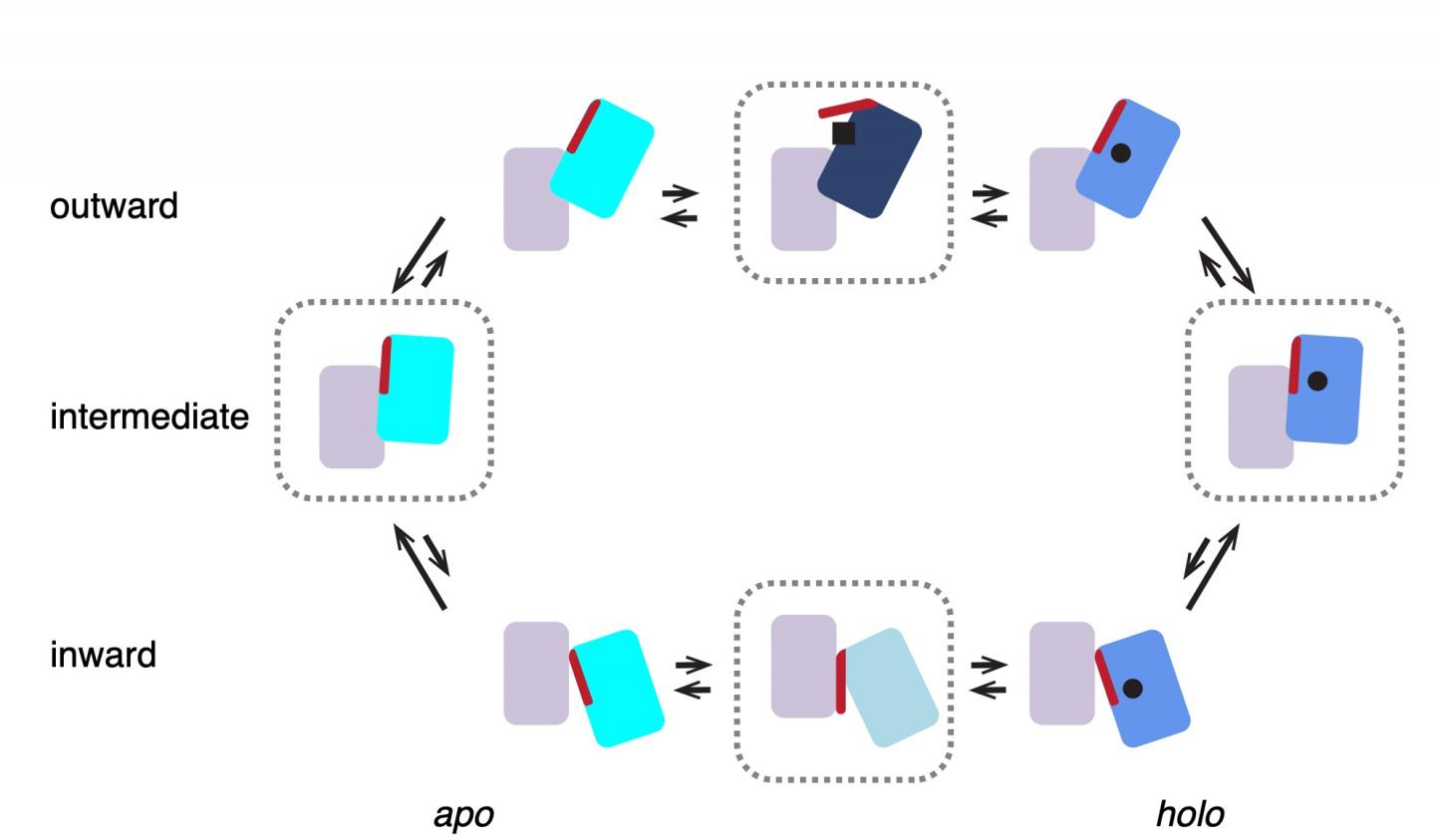
Scheme of the transport cycle of glutamate transporters. Four states enclosed in dashed blocks represent structures observed in this work. GltTk in both apo and holo (Asp) states prefers intermediate…
view more
The structure of a transport complex used by bacteria to import aspartate has been mapped in unique detail by University of Groningen scientists. The proteins were imaged using cryo-electron microscopy. The results reveal that the transporter works very efficiently. This is especially interesting as a similar transporter is vital for signal transduction between human brain cells. The study results were published in Nature Communications on 21 February.
Cells use a myriad of transport proteins to shuttle substances across their membranes: food and building blocks are imported, toxins and other waste is exported. One example is the aspartate transporter, which bacteria use to import this amino acid. The Membrane Enzymology research group led by Professor Dirk Slotboom and the Structural Biology group led by Dr Albert Guskov at the Groningen Institute for Biomolecular Sciences & Biotechnology have studied this transporter for several years, partly because it is a good model for the human transporter that removes the neurotransmitter glutamate from the synaptic cleft, a vital step in the working of our brain cells.
Goods lift
The aspartate transporter in bacterial membranes is a trimer, which means that three identical units are held together tightly to form one complex. Aspartate is picked up from the environment, transported through the cell membrane and released on the inside of the cell. Three sodium ions per unit power this transition, which can be compared to a goods lift: the aspartate and sodium ions bind to part of the transport protein, which then enters the cell. After delivering the aspartate, it exits again.
‘We previously mapped the structure of the complex with aspartate using x-ray crystallography,’ explains Slotboom. These studies showed that the three lifts in the complex were always in the same position. ‘However, biochemical evidence suggested they might work independently of one another.’ That is why he decided to study the transport complex in a more native environment, inside a membrane. This was done using cryo-electron microscopy, a method for creating images of protein complexes.
Questions
The proteins were inserted into small patches of lipid bilayers, kept together by a protein belt. These lipid nanodiscs were quickly frozen and studied in a cryo-electron microscope. By combining a large number of images, the transport complex was imaged at a resolution of 3.2-3.5 angstroms.
‘What we saw was very different from the structures obtained with X-ray crystallography: in most complexes, the lifts were in different positions, consistent with independent movements,’ says Valentina Arkhipova, a postdoctoral researcher in the Slotboom group and first author of the paper. This raises the question of why the protein would form a trimeric complex. Arkhipova: ‘The lift part of each unit requires support to move through the membrane. A single lift anchored inside the membrane might start to wobble. But three lifts with connected anchors form a stable structure.’
Leakage
Another possibility is that the anchor part of the trimer makes the membrane around it a little thinner and less rigid, which makes it easier for the lift to pass through. ‘A monomer would only have this effect on one side, which is energetically less advantageous,’ explains Slotboom. Indeed, studies of lipid nanodiscs containing the transport complex show bending of the bilayer.
The structures also provide an indication of how the transport system prevents leakage of sodium. Slotboom: ‘The lift has a kind of door that, when open, protrudes and prevents the lift from moving.’ The transport process first requires that two sodium ions enter the lift. The negatively charged aspartate can then bind inside, which enables a third sodium ion to enter and bind to the door, closing it. It is therefore impossible for the lift to transport only sodium, which would dissipate the sodium gradient across the membrane driving the transport.
Brains
‘It makes the system very efficient’, says Slotboom. For bacteria, this efficiency may be only a small selective evolutionary advantage. However, for the analogous glutamate transporter in our brains, it is vital. Glutamate is excreted by nerve cells into the synaptic cleft, where it excites the adjacent neuron. After excitation, it has to be removed very quickly and efficiently to reduce noise in signal transmission. Slotboom: ‘For this system, it is vital that there be no leakage.’
###
Reference: Albert Guskov & Dirk J. Slotboom: Structural ensemble of a glutamate transporter homologue in lipid nanodisc environment. Nature Communications 21 February 2020
Simple science summary
Scientists at the University of Groningen studied the structure of a transport system that allows bacteria to pick up aspartate, a building block for proteins, from their environment. There are three identical units in one transport system. Each unit works like a lift that moves up and down through the membrane of the cell. The new study shows that the three lifts work independently and that they are very efficient. The latter is also important for humans: we have a very similar transport system in our brain that plays a vital role in effective communication between brain cells.
Media Contact
Rene Fransen
[email protected]
Original Source
https:/
Related Journal Article
http://dx.