A groundbreaking advancement in the management of treatment-induced dermatologic toxicity has emerged from a recent clinical trial conducted at The University of Texas MD Anderson Cancer Center. This study introduces LUT014, a novel topical gel formulated to mitigate the severity of acneiform rash commonly induced by epidermal growth factor receptor (EGFR) inhibitors used in colorectal cancer therapies. These rashes, which bear significant impact on patients’ quality of life, have historically limited the continuation and dosing of vital targeted treatments. LUT014 represents a pioneering therapeutic modality specifically addressing the underlying molecular pathways responsible for this adverse effect, rather than merely its symptomatic presentation.
Colorectal cancer patients undergoing treatment with EGFR inhibitors such as cetuximab and panitumumab frequently experience substantial cutaneous side effects. The mechanism involves the blockade of the MAP kinase (MAPK) signaling pathway, leading to disruption of normal keratinocyte function and eliciting an inflammatory response manifesting as a painful, acne-like rash predominantly located on the head, neck, and upper trunk. Approximately 75% of patients receiving anti-EGFR therapy develop this rash, which often necessitates dose reduction or cessation of the cancer treatment, consequently compromising therapeutic efficacy and outcomes.
The innovation of LUT014 lies in its targeted inhibition of BRAF kinase, a key regulator within the MAPK pathway. While EGFR blockade suppresses MAPK activity, LUT014 functions to selectively reactivate this signaling cascade within the epidermis, thereby restoring keratinocyte homeostasis and attenuating the inflammatory response that precipitates rash formation. This mechanism-based strategy distinguishes LUT014 from conventional interventions such as topical steroids or systemic antibiotics, which address only inflammation and secondary infections without influencing the primary pathological driver.
Furthermore, LUT014’s formulation as an alcohol-based topical gel ensures minimal systemic absorption, thereby maintaining the integrity of systemic cancer therapies without pharmacokinetic interference. This pharmacological profile is crucial to avoid undermining the efficacy of EGFR inhibitors, a limitation that has compromised prior attempts at managing dermatologic toxicities related to targeted cancer treatments. The topical application allows direct delivery to affected skin sites, optimizing localized therapeutic effects and minimizing adverse events.
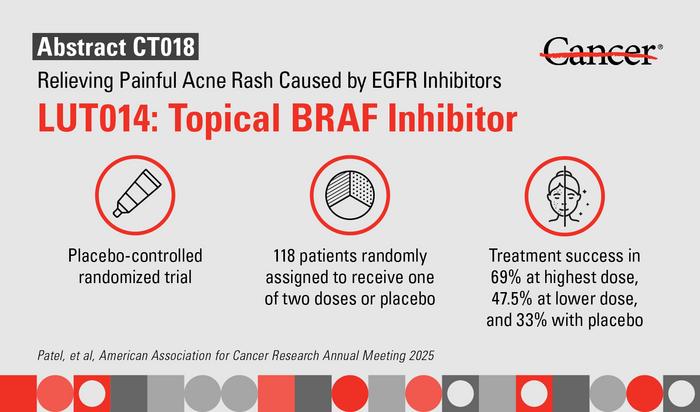
In the randomized, placebo-controlled Phase II clinical trial, 118 colorectal cancer patients experiencing moderate to severe acneiform rash on EGFR inhibitors were enrolled across 23 medical centers. Participants received either 0.1% LUT014, 0.03% LUT014, or a placebo gel over a 28-day period. Treatment success was stringently defined by achieving at least a one-grade reduction in rash severity or an improvement in five or more skin-specific quality-of-life parameters, underscoring the clinical and patient-centered relevance of the outcomes.
Remarkably, treatment success rates with LUT014 were substantially higher compared to placebo, with 69% of patients receiving the 0.1% formulation and 47.5% receiving the 0.03% formulation meeting the criteria, versus only 33% in the placebo group. These findings demonstrate a clear dose-dependent benefit and validate the clinical efficacy of the topical BRAF inhibitor in ameliorating rash severity. Patients also reported enhanced quality of life metrics, reflecting improvements not only in cutaneous symptoms but in broader functional and psychosocial domains.
Adverse events, primarily classified as grade 3 or lower, were relatively mild and consistent with anticipated reactions to topical alcohol-based treatments on inflamed skin. Importantly, the safety profile affirms the tolerability of LUT014, reinforcing its suitability for ongoing patient use without jeopardizing the tolerability of concurrent oncologic regimens. This balance of efficacy and safety addresses a critical unmet need, as long-term use of antibiotics or steroids has been associated with resistance and other complications.
The trial results were formally presented at the American Association for Cancer Research (AACR) Annual Meeting 2025 by Dr. Anisha Patel, associate professor of Dermatology at MD Anderson. Dr. Patel emphasized the clinical imperative for therapies that reduce toxicity burden without compromising cancer control, highlighting LUT014’s novel mechanism as a key advancement that may reshape the supportive care paradigm for patients on targeted agents. She also noted the potential for applying similar strategies to manage toxicities arising from other kinase-targeted cancer treatments.
The research was funded by Lutris Pharma, the developer of LUT014. Disclosure statements reveal collaborative relationships between MD Anderson investigators and various industry partners, underscoring the integrated nature of academic and pharmaceutical collaboration in advancing therapeutic innovations. The detailed list of authors and disclosures is available through the trial’s abstract, facilitating transparency and scientific rigor.
In summary, LUT014’s development marks a significant step forward in supportive oncology care, allowing patients to sustain life-prolonging EGFR inhibitor therapies with improved dermatologic tolerance and quality of life. This clinical advance not only enhances patient experience but may ultimately contribute to better oncologic outcomes by preventing premature treatment interruptions. LUT014 exemplifies how mechanistic insight into adverse effects can yield transformative therapeutic solutions in cancer care.
Subject of Research: Targeted management of EGFR inhibitor-induced dermatologic toxicity in colorectal cancer patients using a novel topical BRAF inhibitor.
Article Title: Novel Topical BRAF Inhibitor LUT014 Mitigates EGFR Inhibitor-Induced Acneiform Rash, Enabling Continued Colorectal Cancer Treatment
News Publication Date: April 27, 2025
Web References:
Clinical trial abstract CT018
MD Anderson Targeted Therapy
Colorectal Cancer Overview
AACR Annual Meeting 2025
MD Anderson AACR Meeting Content
Image Credits: The University of Texas MD Anderson Cancer Center
Keywords: Cancer treatments, Skin disorders, Drug targets, Colorectal cancer, EGFR inhibitors, Side effects, Cell therapies, Inhibitory effects, Kinase inhibitors, Clinical trials, Drug studies, Drug development, Cancer research
Tags: BRAF kinase inhibition in dermatologyclinical trial for skin rash treatmentcolorectal cancer patient support strategiescolorectal cancer therapy advancementsEGFR inhibitors and skin toxicityimproving quality of life for cancer patientsinnovative therapies for cancer-related side effectsLUT014 for acneiform rashmanaging dermatologic toxicity in cancertargeted therapy skin rash solutionstopical gel for cancer treatment side effectstreatment-induced skin rash management