New research demonstrates ability to manipulate nanobubble size, acoustic resonance and, ultimately, ultrasound effectiveness
Credit: Amin Jafari Sojahrood and Al C. de Leon
If you were given “ultrasound” in a word association game, “sound wave” might easily come to mind. But in recent years, a new term has surfaced: bubbles. Those ephemeral, globular shapes are proving useful in improving medical imaging, disease detection and targeted drug delivery. There’s just one glitch: bubbles fizzle out soon after injection into the bloodstream.
Now, after 10 years’ work, a multidisciplinary research team has built a better bubble. Their new formulations have resulted in nanoscale bubbles with customizable outer shells — so small and durable that they can travel to and penetrate some of the most inaccessible areas in the human body.
The work is a collaboration between Al C. de Leon and co-authors, under the supervision of Agata A. Exner of the Department of Radiology at the Case Western Reserve University School of Medicine in Cleveland and Amin Jafari Sojahrood under the supervision of Michael Kolios of the Department of Physics at Ryerson University and the Institute for Biomedical Engineering, Science and Technology (iBEST) in Toronto. Their results were recently published in ACS Nano, in a paper entitled “Towards Precisely Controllable Acoustic Response of Shell-Stabilized Nanobubbles: High-Yield and Narrow-Dispersity”.
“The advancement can eventually lead to clearer ultrasound images,” says Kolios. “But more broadly, our joint theoretical and experimental findings provide a fundamental framework that will help establish nanobubbles for applications in biomedical imaging — and potentially into other fields, from material science to surface cleaning and mixing.”
Bubbles in Ultrasound: Shrinking Down to Nanoscale
Ultrasound is the second most used medical imaging modality in the world. As with other modalities, a patient may swallow or be injected with an agent to create image contrast, thereby making bodily structures or fluids easier to see.
With ultrasound, bubbles serve as the contrast agent. These gas-filled globes are enclosed by a phospholipid shell. Contrast is generated when ultrasound waves interact with the bubbles, causing them to oscillate and reflect soundwaves that differ significantly from waves reflected by body tissues. Bubbles are used routinely in patients to improve image quality and enhance the detection of diseases. But due to their size (about the same as red blood cells), microbubbles are confined to circulating in blood vessels, and cannot reach diseased tissue outside.
“Our research team at CWRU now engineered stable, long-circulating bubbles at the nanoscale — measuring 100-500 nm in diameter,” says Exner. “They’re so that they can even squeeze through leaky vasculature of cancerous tumours.”
With such capabilities, nanobubbles are well-suited for finer applications such as molecular imaging and targeted drug delivery. Working together with the Ryerson team, the researchers have developed a clearer understanding of the theory of how nanobubbles are visualized with ultrasound, and what imaging techniques are needed to best visualize the bubbles in the body.
Controlling Nanobubble Behaviour
Size issues aside, bubbles are also complex oscillators, exhibiting behaviours that are difficult to control. In the current work, the research team also devised a way to precisely control and predict how bubbles interact with and respond acoustically to ultrasound.
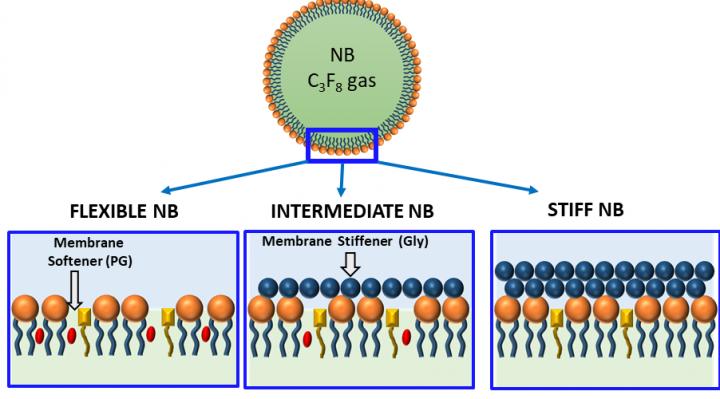
“By introducing membrane additives to our bubble formulations, we demonstrated the ability to control how stiff (or how flexible) the bubble shells become,” says de Leon. “Bubble formulations can then be customized to match the particular needs of different applications.”
For example, stiffer, stable bubble designs may last long enough to reach body tissues that are difficult to access. Softer bubbles may produce clearer ultrasound images of certain types of body tissue. Bubble oscillation could even be tweaked to increase cell permeability, potentially increasing drug delivery to diseased cells, which may in turn decrease the dosage required.
Patients, the Ultimate Beneficiaries
Having successfully demonstrated the ability to customize bubble shell properties and their interaction with sound waves, the current work has exciting implications for nanobubble potency — in both diagnostic and therapeutic applications.
Sojahrood sees many potential benefits, for biomedicine and for patients in clinic. “Compared to other imaging or treatment options, such as surgery with scalpels, bulky MRI machinery, or the risk of radioactive iodine in CT scans, ultrasound could be a lot faster, cheaper, more effective and less invasive,” he says. “By advancing ultrasound through nanobubbles, we could eventually make diagnosis and treatment more available and more effective, even in more remote areas of the world, ultimately improving patient outcomes and saving more lives.”
###
Research reported in this publication was supported by:
National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award number R01EB025741
Office of the Assistant Secretary of Defense for Health Affairs, through the Prostate Cancer research program under award no. W81XWH-16-1-0371
Case Western Reserve University Coulter Translational Partnership
A.J.S. was supported by a CIHR Vanier scholarship
M.C.K. and A.S. received support from the CIHR and NSERC
P.W. and E.P. received financial support from NSF CAREER award no. 1551943. A.d.L
A.E. would like to acknowledge the help from Olive Jung
Media Contact
Suzana Pinto
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




