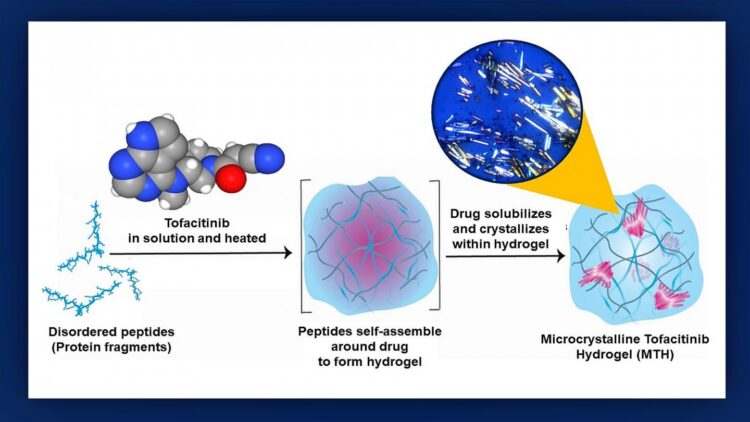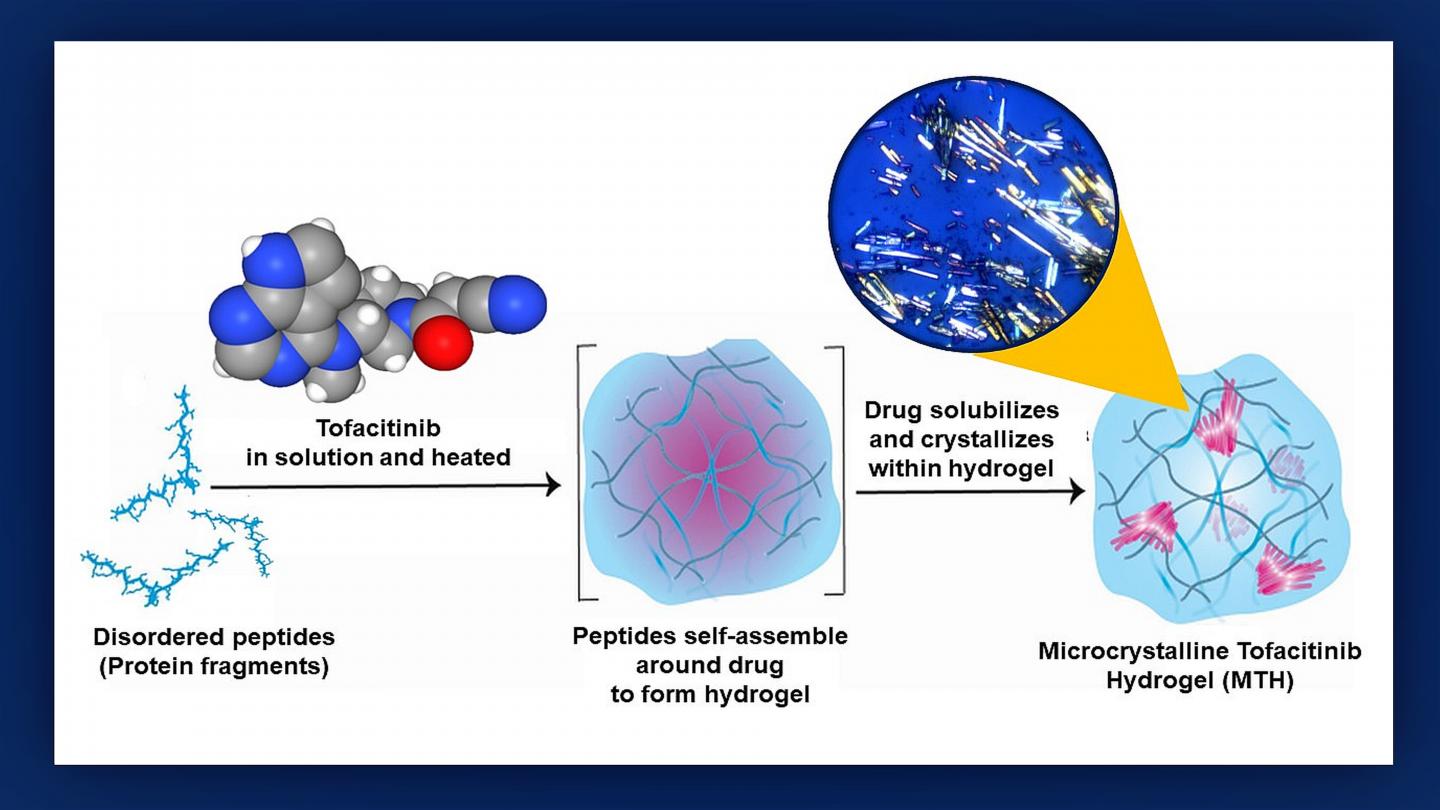
Credit: Graphic created by M.E. Newman, Johns Hopkins Medicine, using an original illustration by Poulami Majumder and tofactinib molecular model courtesy of the National Center for Biotechnology Information
For patients who receive a heart transplant in the near future, the old adage, “Good things come in small packages,” may become words to live by. In a recent study, researchers at Johns Hopkins Medicine and the National Cancer Institute (NCI) demonstrated in mice that they can easily deliver a promising anti-rejection drug directly to the area surrounding a grafted heart by packaging it within a tiny three-dimensional, protein gel cocoon known as a hydrogel. Best of all, the researchers say that the release of the drug is spread out over time, making it highly regulatable and eliminating the need for daily medication to keep rejection in check.
The findings are presented in the Aug. 18, 2020, issue of the journal Small.
Preventing the rejection of a transplanted heart has often been a Catch-22 situation. If you give an organ recipient large amounts of immune suppression drugs, there may be serious side effects, including kidney damage, hypertension, blood sugar imbalances and even lymphomas. Lowering the dose may be safer for general health but increases the risk that rejection will not be properly controlled and the grafted heart will be lost.
“What was needed was a drug delivery method that would get the anti-rejection medication only where needed; protect the drug from premature degradation; and maintain a high concentration for the period of time needed to retrain the immune system,” says study co-author Giorgio Raimondi, M.Sc., Ph.D., assistant professor of plastic and reconstructive surgery at the Johns Hopkins University School of Medicine.
“Two earlier studies used the hydrogel method to successfully deliver conventional immunosuppressive drugs to other sites, and this prompted us to try it out for transplanted hearts,” Raimondi explains. “Additionally, work by a team at the NCI Frederick National Laboratory for Cancer Research under Joel Schneider [Ph.D., a study co-author] showed that hydrogel-drug packages can be administered by syringe.”
The drug these researchers wanted to supply to transplanted hearts is tofacitinib, an inhibitor of the process by which cells alert their receptiveness to binding with inflammation-inducing proteins called cytokines. In normal immune responses to foreign invaders within the body, cytokines play a critical role in alerting specialized white blood cells — T lymphocytes — to attack and remove the threatening bacteria or viruses. However, cytokines in the presence of a transplanted heart can direct the immune system to destroy the graft.
To see if a hydrogel courier could be used to deliver tofacitinib, the researchers first grafted mouse hearts into the necks of recipient mice to create an animal model of a human transplant. Next, they mixed tofacitinib with a solution of small protein fragments that assembled themselves around the drug during a 24-hour incubation process that Raimondi likens to the “make your own crystal” kits popular with children.
“We found that making tofacitinib into a crystal best controlled how the drug spreads out from the hydrogel,” says study lead author Poulami Majumder, Ph.D., former NCI at Frederick postdoctoral fellow. “The resulting ‘microcrystalline tofacitinib hydrogel,’ or MTH, was extremely stable, preserved the encapsulated drug in pristine condition and could be injected at the transplantation site simply by using a syringe.”
The researchers tested the MTH delivery system in their mouse model in tandem with a second immunosuppressant drug, CTLA4-Ig, which was injected separately. This was the first time that this specific combination therapy had been tried.
To determine if the location of MTH delivery was important, the researchers injected the packaged medicine locally, at the transplant site, and distantly, near the mouse’s tail. As expected, only the group of mice with local injections showed a significant increase of graft survival time.
“The average survival of the grafted hearts in the locally injected group was approximately 125 days compared with just 35 days for mice injected with MTH far from the transplant,” Raimondi says. “We also tested the plasma of the former group and found only minimally detectable traces of tofacitinib — meaning that MTH delivery keeps the drug close to the transplant site and enables it act synergistically with CTLA4-Ig to provide enhanced and lasting protection of the organ.”
Without the tofactinib/CTLA4 treatment, Raimondi says, the transplanted mouse hearts stopped beating within 10 days.
Raimondi says that among the advantages to using MTH as a drug-delivery system are that the hydrogel releases its content slowly, over a period of 5 to 20 days, and does not cause other complications because it is biocompatible, noninflammatory and biodegradable. He and his colleagues believe that using crystal engineering to further improve the hydrogel capsule, more control over the release rate can be obtained — a critical goal to meet before human trials can be attempted — or, the capsule can be made “tunable” to deliver drug only when the grafted heart is attacked by the immune system.
The researchers also feel that with additional research and testing, the MTH delivery system could be applied to fighting rejection of transplanted organs other than the heart and in the treatment of autoimmune diseases.
###
Along with Raimondi, the other study team members from the Johns Hopkins University School of Medicine are Yichuan Zhang; Marcos Iglesias, D.V.M., Ph.D.; Byoung Chol Oh, Ph.D.; Georg Furtmüller, M.D.; and Gerald Brandacher, M.D. Members from NCI, along with Schneider and Majumder, are James Kelly, Ph.D.; Christopher Lai, Ph.D.; Lixin Fan, Ph.D.; Jason Stagno, Ph.D.; Yun-Xing Wang, Ph.D.; and Caroline Andrews, D.V.M. Nimit Patel, of Leidos Biomedical Research Inc., also participated in the research effort.
The study was supported by NCI’s Center for Cancer Research Intramural Research Program; the Department of Plastic and Reconstructive Surgery at the Johns Hopkins University School of Medicine; and the Department of Defense’s U.S. Army Medical Research Acquisition Activity.
Raimondi, Schneider and Majumder have a patent pending (PCT/US2019/030656) for “peptide hydrogels for delivery of immunosuppressive drugs and uses thereof.”
Media Contact
Michael Newman
[email protected]
Original Source
https:/
Related Journal Article
http://dx.