Credit: Stobart et al Nature Communications (2016).
Crafting a vaccine against RSV (respiratory syncytial virus) has been a minefield for 50 years, but scientists believe they have found the right balance.
Researchers at Emory University School of Medicine and Children's Healthcare of Atlanta report that they have engineered a version of RSV that is highly attenuated – weakened in its ability to cause disease – yet potent in its ability to induce protective antibodies. Used as a vaccine, this engineered virus can protect mice and cotton rats from RSV infection.
The results are scheduled for publication in Nature Communications on December 21.
"Our paper shows that it's possible to attenuate RSV without losing any immunogenicity," says senior author Martin Moore, PhD, associate professor of pediatrics at Emory University School of Medicine and a Children's Healthcare of Atlanta Research Scholar. "This is a promising live-attenuated vaccine candidate that merits further investigation clinically."
The next steps for this vaccine are to produce a clinical grade lot and conduct a phase 1 study of safety and immunogenicity in infants, Moore says.
RSV is the number one cause of pneumonia deaths in children worldwide and one of the top causes of infant hospitalization in the United States. Most children in the USA become infected in the first year of life.
Even so, there is no vaccine against RSV available. In the 1960s, an attempt to develop an RSV vaccine by chemically inactivating the virus, like in a standard flu shot, backfired. Exposure to this vaccine actually made natural RSV infection in infants worse. More recently, a clinical trial of a protein-only RSV vaccine in older adults showed disappointing results.
Several successful vaccines against other viruses, likes measles, polio, and mumps, have been created by weakening the viruses in cell culture. A strain that is safe enough for use as a vaccine, but still stimulates the immune system, is supposed to result.
"Achieving that balance is a challenge," Moore says. "Weakening RSV by passage hasn't worked out satisfactorily. It turns out that for RSV, the natural virus does not induce a lot of immunity itself."
Instead, Moore and his colleagues engineered RSV to enhance production of a key protein, called F (for fusion). F is critical for RSV's ability to enter cells and is the target of several vaccines in development. By making mutations in the viral gene that encodes F, the researchers also made the virus more stable with respect to heat, which could help vaccine manufacturing and distribution.
At the same time, the researchers removed or weakened several other viral genes that promote infection and suppress the immune system. One measure of this is that the modified virus, called OE4, stimulates high levels of antibody production.
Moore and his team rewrote several of the viral genes in the OE4 strain using a process called codon-deoptimization, which makes it less likely for a re-engineered virus to mutate back to its original form.
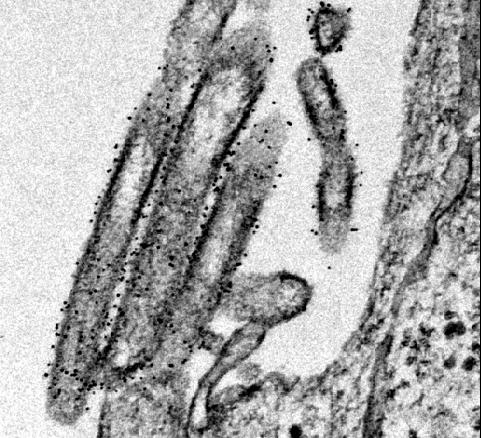
When examined via electron microscopy, OE4 virions looked similar to standard RSV virions, except that OE4 had higher levels of the immunogenic form of the F protein and lower levels of another protein called G. The electron microscopy work was a collaboration with Elizabeth Wright, PhD, Emory associate professor of pediatrics and co-senior author on the paper, and her colleagues at Emory's Robert P. Apkarian Integrated Electron Microscopy Core.
OE4 vaccination could completely protect mice and cotton rats against later infection by standard forms of RSV. In contrast with a chemically inactivated RSV vaccine, OE4 did not result in enhanced lung disease in cotton rats.
Christopher Stobart, PhD, now assistant professor of biology at Butler University, and instructor Christina Rostad, PhD are co-first authors of the paper. Cotton rat studies were performed at Baylor College of Medicine and at Sigmovir Biosystems.
Emory has optioned the vaccine technology to a startup company, Meissa Vaccines, Inc. Moore co-founded and serves as chief scientific officer for Meissa Vaccines, Inc. Moore, Stobart, Rostad, and co-authors Anne Hotard and Jia Meng are co-inventors in a patent application describing the RSV vaccine reported in the Nature Communications paper. Emory and Moore could benefit financially from this technology, and this relationship has been reviewed and approved by Emory University School of Medicine.
###
The research was supported by the National Institute of Allergy and Infectious Diseases (R01AI087798, U19AI095227, T32AI074492), the Atlanta Pediatric Scholars program (K12HD072245) and a pilot grant from the Emory/Children's/Georgia Tech Center for Childhood Infections and Vaccines.
Media Contact
Quinn Eastman
[email protected]
404-727-7829
@emoryhealthsci
http://whsc.emory.edu/home/news/index.html
############
Story Source: Materials provided by Scienmag





