Anew study by the Neuronal Dynamics Research Group at UPF has elucidated the cellular and molecular mechanisms that regulate the balance between the differentiation of neurons and the maintenance of progenitor cells during the construction of the embryonic brain. The study, conducted in the zebrafish model, has been published in the journal Cell Reports.
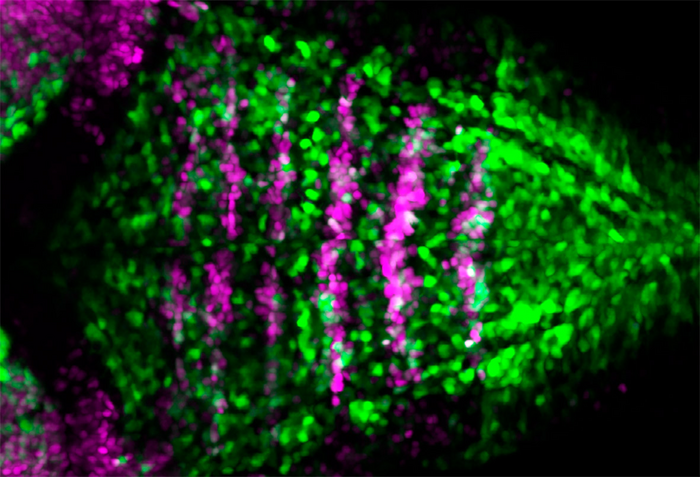
Credit: UPF authors
Anew study by the Neuronal Dynamics Research Group at UPF has elucidated the cellular and molecular mechanisms that regulate the balance between the differentiation of neurons and the maintenance of progenitor cells during the construction of the embryonic brain. The study, conducted in the zebrafish model, has been published in the journal Cell Reports.
In vertebrates, the central nervous system is formed from an embryonic structure divided into three vesicles of the brain and the spinal cord. The hindmost vescile is called the rhombencephalus and is preserved in all vertebrates. It will result in essential adult derivates for the regulation of breathing, heart rate, etc. During embryonic development, the posterior brain is subdivided into seven segments, called rhombomeres.
During embryonic development, coordination takes place between the proliferation of progenitor stem cells responsible for the organ’s growth and its differentiation into neurons. For this reason, this ability to give rise to neurons is localized to specific sites.
Now, the group led by Cristina Pujades at the UPF Department of Medicine and Life Sciences (MELIS) has investigated how rhombomeric boundary cells give rise to neurons while maintaining a proliferative reservoir of progenitor stem cells. “In the initial stages of embryo formation it is important to maintain a number of stem cells that allow the formation of neurons later on”, sets out Covadonga F. Hevia, first author of the study.
Cristina Pujades, study coordinator: “during brain formation, the processes of proliferation and differentiation must be maintained, they must be regulated very precisely, and each step must occur at the right time. This coordination is crucial because any problems during the process can result in neural disorders”.
They do this by asymmetric divisions, in other words, when a progenitor cell divides, it gives a daughter cell that becomes a neuron and stops dividing, and another daughter cell that remains a progenitor. This allows retaining the stem cell reservoir over time. “In this work we have discovered that the asymmetric cell division triggered by the so-called Notch signalling pathway allows rhombomeric boundary cells to form neurons while maintaining stem cells for later on”, she adds.
“The cells express markers, but, as they vary during the process, when they ceased to express them we lost track of them and we could no longer follow them. Now, the CRISPR technique allows us to insert a reporter gene and generate a transgenic zebrafish line where the cells we are interested in express a fluorescent marker. We can thus observe them, and follow them over time, see how they divide, what type of neurons they give rise to, etc.”, explains Carolyn Engel-Pizcueta, author of the article.
In the words of Cristina Pujades, study coordinator: “during brain formation, the processes of proliferation and differentiation must be maintained, they must be regulated very precisely, and each step must occur at the right time. This coordination is crucial because any problems during the process can result in neural disorders”, Pujadas concludes.
Reference article:
Hevia, CF, Engel-Pizcueta, C, Udina, F and Pujades C. The neurogenic fate of the hindbrain boundaries relies on Notch3-dependent asymmetric cell divisions. Cell Reports, 7 June 2022. DOI: https://doi.org/10.1016/j.celrep.2022.110915
Journal
Cell Reports
DOI
10.1016/j.celrep.2022.110915
Method of Research
Experimental study
Subject of Research
Animals
Article Title
The neurogenic fate of the hindbrain boundaries relies on Notch3-dependent asymmetric cell divisions
Article Publication Date
7-Jun-2022




