Researchers from Japan Advanced Institute of Science and Technology (JAIST) have successfully established a new humidity in situ measurements for anion conducting polymer thin films
Credit: Yuki Nagao from JAIST
Ishikawa, Japan – As decarbonization progresses rapidly in the world, fuel cells offer potentially higher electrical efficiency than conventional power-generating systems. Anion exchange membrane fuel cells offer advantages of using non-precious metal catalysts than proton exchange membrane fuel cells. One of the challenges of this next-generation fuel cell is to clarify the hydroxide ion conductivity in the ion conductive polymer around the electrode catalyst. The difficulty of studying the hydroxide ion conductivity at the electrode interface is that the hydroxide ion, which is a carrier, easily reacts with carbon dioxide in the air. To solve this problem, all evaluation devices were improved so that the sample did not come into contacting with air.
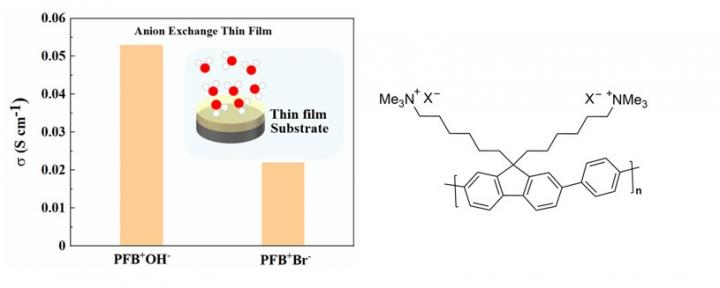
In a new study published in ChemSusChem (Accepted Article), researchers from Japan Advanced Institute of Science and Technology (JAIST), namely Associate Professor Yuki Nagao and Ph.D. students Fangfang Wang and Dongjin Wang, succeeded in precisely identifying the hydroxide ion conductivity and the water amount contained in the sample without exposing the thin film sample to air. Fluorene-based cationic polymers were synthesized, and Br– and OH– samples as counter anions were prepared for comparison. It was revealed that the 270-nm-thick thin film containing hydroxide ions exhibits a high hydroxide ion conductivity of 0.05 S cm-1. This ionic conductivity was more than twice as high as the value of the thin film containing Br– ions as shown in Figure 1.
Surprisingly, it was also revealed that the hydroxide ion conductivity of the thin film form containing hydroxide ions was comparable to that of the thick membrane form. This tendency seems to be different from the results reported in proton conductive polymers.
“Developing a better understanding of these properties and their impacts on hydroxide ion conduction will be important for both clarifying hydroxide ion conduction mechanisms and improving fuel cell performance,” explains Associate Professor Yuki Nagao from JAIST. New findings seem to be a solid step toward a hydrogen society.
###
Funding information
This study was funded by Grant-in-Aid for KAKENHI grants (JP18K05257 and JP21H01997), a Grant-in-Aid for Scientific Research on Innovative Areas “hydrogenomics “project (JP21H00020) from Japan Society for the Promotion of Science (JSPS).
Media Contact
Yuki Nagao
[email protected]
Related Journal Article
http://dx.




