Living cells compete with each other and try to adapt to the local environment. Cells that are unable to do so are eliminated eventually. This cellular competition is crucial as the surrounding normal epithelial cells use it to identify and eliminate mutant cancer cells. Studies have reported that when activating mutants of “Ras” proteins are expressed in mammalian epithelial cells, they are pushed toward the lumen, excreted along with other bodily waste, and eliminated by competition. Epithelial cells containing Ras mutants have been reported to be removed in this manner in several organs, including the small intestine, stomach, pancreas, and lungs. This suggests that cell competition is an innate defense system orchestrated by epithelial cells to prevent the accumulation of incidentally produced cancerous cells and thereby suppress cancer formation.
Credit: Shunsuke Kon of Tokyo University of Science (TUS), Japan
Living cells compete with each other and try to adapt to the local environment. Cells that are unable to do so are eliminated eventually. This cellular competition is crucial as the surrounding normal epithelial cells use it to identify and eliminate mutant cancer cells. Studies have reported that when activating mutants of “Ras” proteins are expressed in mammalian epithelial cells, they are pushed toward the lumen, excreted along with other bodily waste, and eliminated by competition. Epithelial cells containing Ras mutants have been reported to be removed in this manner in several organs, including the small intestine, stomach, pancreas, and lungs. This suggests that cell competition is an innate defense system orchestrated by epithelial cells to prevent the accumulation of incidentally produced cancerous cells and thereby suppress cancer formation.
In general, mutations in multiple genes accumulate in a stepwise manner when normal cells become cancerous. However, it is not known how cell competition is affected by this process. For instance, human colorectal cancer develops when the adenomatous polyposis coli (APC) gene becomes dysfunctional and activates “Wnt signaling,” followed by the activation of Ras signaling.
In a recent study, a team of researchers from Japan, led by Associate Professor Shunsuke Kon of the Department of Cancer Biology, Institute of Biomedical Research and Innovation, Tokyo University of Science (TUS), examined the effects of the accumulation of stepwise gene mutations on cell competition and investigated the role of cell competition in the actual cancer formation process. Their study was published in Nature Communications on November 3, 2023 with Mr. Kazuki Nakai, a third year PhD student at the Graduate School of Life Sciences in TUS, as the lead author.
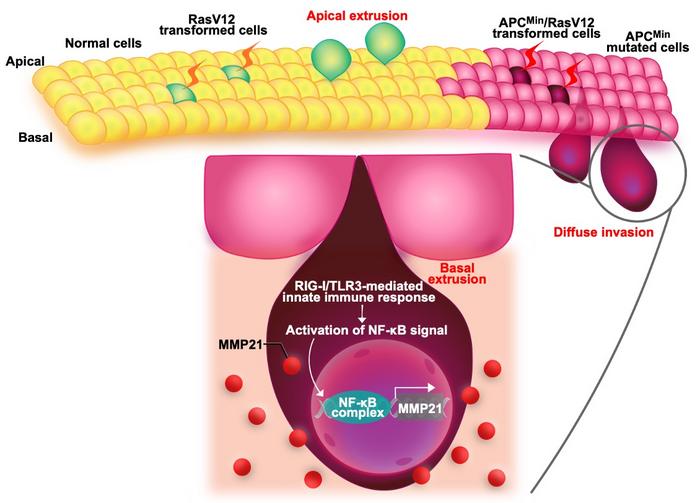
The study results showed that when Wnt signals were activated in epithelial cells, cell competition function was altered. Activated Ras mutant epithelial cells, which would normally be eliminated into the lumen, instead infiltrated diffusely into the tissue to form highly invasive cancerous tumors.
As senior author Dr. Kon explains, “We discovered that in epithelial tissues where Wnt and Ras signals, which commonly occur in human colorectal cancer, are activated in stages, the function of cell competition is altered. It was revealed that the production of cancer cells that diffusely infiltrate into the interstitium is promoted.”
Further, the research team identified an increased expression of matrix metalloproteinase 21 (MMP21) as one of the mechanisms underlying the production of diffusely invasive cancer cells in early colorectal cancer due to abnormal cell competition. This, in turn, was shown to be directly caused by activation of nuclear factor kappa B (NF-κB) signals via the innate immune system. Blocking NF-κB signaling restored the luminal elimination of Ras mutant epithelial cells. These findings raise some intriguing questions, such as “How do transformed cells sense the cellular content that leads to the NF-B-MMP21 pathway?” and “How do surrounding cells recognize transformed cells and prepare them for cellular extrusion?” These questions will almost certainly need to be addressed in the future.
The results of this research show that cancer cells with accumulated, sequential genetic mutations, alter the function of cell competition and use it to enhance their own invasive ability. Instead of being eliminated to the lumen, they infiltrate into the tissue, producing high-grade cancer cells. While the research team did note that the cancer histopathology of the mice used in this study resembled diffuse-type cancer in humans, future research is needed to determine whether the NF-κB-MMP21 pathway is relevant to other cancers. For instance, investigating scirrhous gastric cancer, a typical diffuse-type cancer, would be particularly interesting.
Overall, these findings demonstrate that Wnt activation disrupts cell competition, and confers invasive properties on transformed cells to escape primary epithelial sites. Understanding how the molecular landscape is re-modeled to change the fate of cancer cells with high mutational burdens could be used for therapeutic purposes. This could be of interest to researchers focused on Wnt signaling or cancer research, such as those at the Koch Institute for Integrative Cancer Research at MIT and Cancer Research UK, who are working towards common goals.
Dr. Kon concludes by saying, “This study further brings forth the prospect that cell competition constrains the order of appearance of mutations during tumor development, highlighting a link between cell competition and carcinogenesis. We hope that this will pave the way for the development of new cancer treatments from the standpoint of cell competition and infiltration for the benefit of our society.”
***
Reference
DOI: https://doi.org/10.1038/s41467-023-42774-6
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society,” TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https://www.tus.ac.jp/en/mediarelations/
About Dr. Shunsuke Kon from Tokyo University of Science
Dr. Shunsuke Kon is a Junior Associate Professor in the Cancer Biology Department of the Research Institute for Biomedical Sciences. He obtained his Ph.D. from the Tohoku University Graduate School of Life Sciences in 2008. He was previously associated with the Institute of Genetic Medicine at Hokkaido University. His primary research interest has been in the field of tumor biology. He has more than 20 publications to his credit. In addition, he has received the Best Articles of the Year award.
Journal
Nature Communications
DOI
10.1038/s41467-023-42774-6
Method of Research
Experimental study
Subject of Research
Lab-produced tissue samples
Article Title
Wnt activation disturbs cell competition and causes diffuse invasion of transformed cells through NF-κB-MMP21 pathway
Article Publication Date
3-Nov-2023
COI Statement
Akihiro Okashi is currently a part-time employee of Astellas Pharma Inc. through a cross-appointment system and was previously employed by Takeda Pharmaceutical Company, Ltd. He has reported paid consulting or advisory roles for Ono Pharmaceutical Company Ltd., Craif Inc., and GEXVal Inc., which are not relevant to this study. The remaining authors declare no competing interests.


