In a remarkable leap forward for biomedical imaging, a research team from Nanjing University of Science and Technology (NJUST), led by Professor Chao Zuo, has unveiled an innovative AI-empowered super-resolution microscopy technique that significantly advances the visualization of live cellular dynamics. This pioneering method, known as ensemble deep learning-enabled single-shot composite structured illumination microscopy (eDL-cSIM), captures intricate cellular structures from a solitary camera exposure, marking a transformative stride toward faster, less invasive imaging with unparalleled clarity. The groundbreaking work is detailed in the forthcoming issue of the journal PhotoniX.
Conventional super-resolution fluorescence microscopy techniques have indeed redefined cellular observation by breaching the optical diffraction barrier. However, the bottlenecks remain substantial. Many existing modalities necessitate capturing dozens, if not hundreds, of sequential frames to computationally reconstruct a high-resolution image. Such demands translate into prolonged exposure times, amplified risks of photobleaching fluorescent markers, and motion-induced artifacts, all of which undermine image fidelity and live-cell experimentation. Moreover, reliance on post-acquisition image processing can introduce reconstruction errors, especially under conditions of low photon flux and compromised signal-to-noise ratios (SNR).
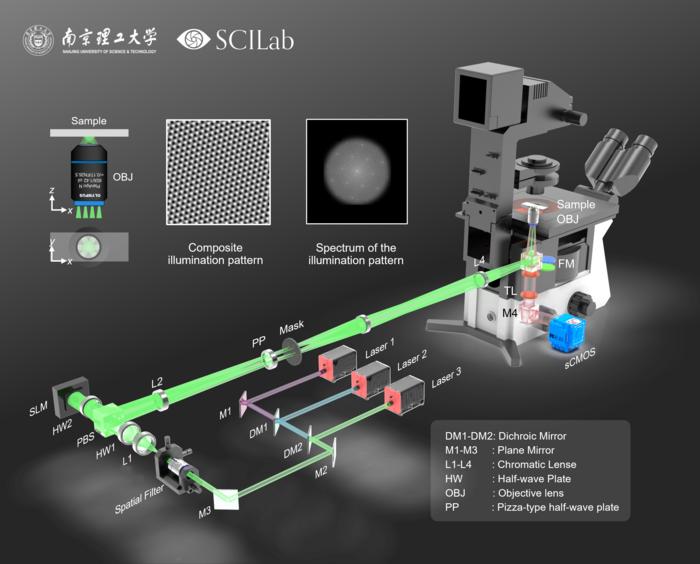
The NJUST team’s eDL-cSIM method addresses these challenges head-on by embedding a six-beam interference pattern into a single exposure frame. This approach efficiently encodes multidirectional super-resolution spatial frequencies simultaneously, effectively compressing data acquisition time while significantly reducing photon dosage. Central to this technique is an ensemble neural network architecture that fuses Transformer modules with multi-model integration strategies, enabling precise decoding of intricate illumination patterns. This neural ensemble reconstructs images with a lateral resolution near 100 nanometers from just one shot, a capability that traditionally demanded multiple exposures.
Professor Zuo emphasizes that this innovation is not simply an iterative improvement but represents a paradigm shift. By merging physical optics and artificial intelligence, eDL-cSIM combines the robustness of structured illumination microscopy with the adaptability and interpretive power of deep learning algorithms. This confluence yields superior image reconstruction speed and quality, minimizes phototoxic effects, and is resilient to environmental perturbations that typically challenge live-cell microscopy.
Beyond mitochondrial observations, eDL-cSIM exhibits remarkable generalization capabilities across varied cellular structures and sample types. This versatility is critical for biomedical applications, allowing researchers to study a broad spectrum of organelles and molecular phenomena with equal precision and efficiency. The system’s environment-robust design ensures stability even under fluctuating laboratory conditions, addressing a common impediment faced by sensitive optical imaging setups.
From a technical perspective, the innovation is anchored in a novel optical configuration that simultaneously projects six spatially and angularly distinct illumination beams. This ensemble produces composite interference patterns encoding rich spatial information which, until now, required multiple sequential acquisitions to decode. By integrating this with an ensemble deep learning model that includes Transformer-based attention mechanisms, the method adeptly dissects the complex interference signatures to reconstruct super-resolved images from highly compressed data.
This approach’s implications are broad-reaching. By dramatically reducing the requirement for multiple frames and high photon doses, eDL-cSIM enables prolonged live-cell imaging without inducing phototoxic damage or photobleaching. This gentler microscopy offers unprecedented windows into cellular physiology, making it a potentially indispensable tool in fields ranging from developmental biology and neuroscience to pharmacology and personalized medicine. It paves the way for continuous, high-fidelity observation of cellular processes on timescales and at resolutions previously considered technically and biologically prohibitive.
Furthermore, the development highlights the transformative role of artificial intelligence in modern microscopy. The fusion of physical imaging techniques with advanced computational models is ushering in an era of “intelligent microscopes” capable of optimizing data acquisition and enhancing image reconstruction in real time. This AI-aided microscopy transcends the classical trade-offs between speed, resolution, and photodamage, setting new standards in live-cell imaging.
The implications for drug discovery are equally profound. Fast and accurate imaging of live cells can accelerate phenotypic screening, help elucidate mechanisms of drug action, and support the identification of early cellular responses to therapeutic agents. As cellular dynamics become more accessible through such technologies, researchers can unravel pathological processes with greater clarity, enabling more targeted and efficacious interventions in complex diseases.
In conclusion, eDL-cSIM emerges as a groundbreaking methodology that capitalizes on the integration of structured illumination microscopy and innovative deep learning frameworks to deliver rapid, high-resolution, and minimally invasive imaging. This breakthrough opens new frontiers for observing cellular microenvironments and paves the way for discoveries that may redefine our understanding of health and disease at the microscopic scale. As Professor Zuo and his team continue to refine the technology, the scientific community anticipates broader adoption of intelligent microscopy systems across diverse biomedical research arenas.
Subject of Research: Cells
Article Title: Ensemble deep learning-enabled single-shot composite structured illumination microscopy (eDL-cSIM)
News Publication Date: 7-May-2025
Web References:
http://dx.doi.org/10.1186/s43074-025-00171-w
Image Credits: Jiaming Qian
Keywords: AI-driven microscopy, super-resolution imaging, live-cell imaging, deep learning, structured illumination microscopy, mitochondrial dynamics, phototoxicity reduction, composite interference patterns, Transformer neural networks, biomedical imaging innovation
Tags: AI-powered imaging technologybiomedical imaging advancementsdrug development imaging technologiesensemble deep learning microscopylive cellular dynamics visualizationmitochondrial dynamics observationneurodegeneration research methodsphototoxicity in live-cell imagingreal-time cellular behavior analysissingle-shot learning in imagingstructured illumination microscopy techniquessuper-resolution microscopy