In summer 2011, University of Colorado Cancer Center investigators Joaquín Espinosa, PhD, and Matthew Galbraith, PhD, taught a summer symposium on gene expression at Cold Spring Harbor Laboratory in Long Island, New York. As part of the three-week course, one of their students, Joel Perrez-Perri from Dr. Pablo Wappner’s lab at the Instituto Leloir in Buenos Aires, Argentina, presented data from experiments on fruit flies describing the role of the histone acetyl-transferase TIP60 (aka KAT5) in regulating the expression of genes controlled by a protein known as HIF1A. Now five years later, in summer 2016, studies resulting from this seemingly obscure finding have resulted in a paper published today in the high-impact journal Cell Reports showing the role of TIP60 in allowing human colorectal cancer cells to survive at the oxygen-poor centers of tumors.
“Tumors often can’t grow the new blood vessels needed to supply themselves with the oxygen that most tissues would need to grow. In order to survive in low oxygen – in conditions of hypoxia – tumors produce the protein HIF1A. In human tumors, hypoxia and high expression of HIF1A are both predictors of bad outcomes,” says Galbraith, now an Instructor of Pharmacology at the CU School of Medicine.
If it were possible to silence HIF1A, many cancers would succumb to hypoxia. Unfortunately, it has proven difficult to find drugs that inhibit the function of HIF1A. To circumvent this problem, scientists around the world are performing research to identify auxiliary proteins inside the cell required for HIF1A activity – referred to as cofactors – that could be more amenable to pharmacological inhibition.
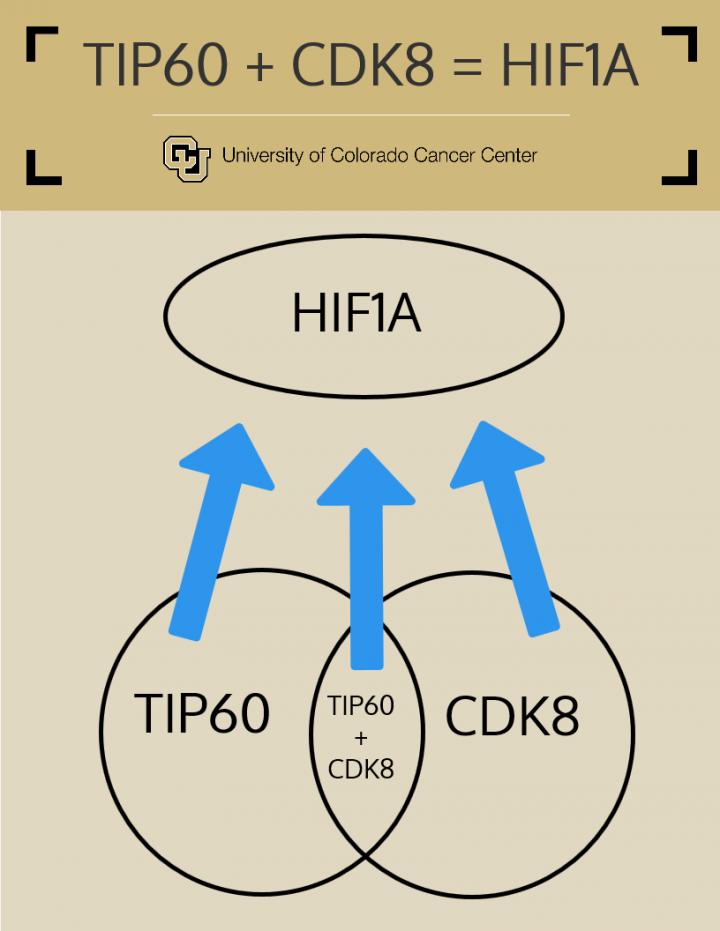
At the time of the serendipitous summer encounter, the labs led by Espinosa in Colorado and Wappner in Buenos Aires were employing vastly different approaches to identify HIF1A cofactors. While Galbraith and Espinosa used human cancer cells grown in petri dishes, Perez-Perri and Wappner employed fruit flies, an organism frequently used as a model due to ease of genetic manipulation. Efforts in the Espinosa lab led to the identification of an enzyme known as CDK8 that is required for much of HIF1A activity in cancer cells. Efforts in the Wappner lab led to the identification of TIP60, also an enzyme, required for HIF1A activity in flies. The power of these discoveries resides in the fact that enzymes are a type of proteins whose activities can be more easily manipulated with medicines (unlike the action of the gene HIF1A itself). In fact, such drugs are already available to shut down the activity of CDK8 and TIP60.
In 2013, Galbraith and Espinosa published their findings in the prestigious journal Cell and embarked on a collaboration with the scientists in Buenos Aires to investigate whether TIP60’s role in the response to hypoxia in flies was conserved in human cancer cells.
“It was a nice coincidence and a great opportunity to collaborate with fellow scientists in Argentina, the country where I was born, raised and received my education,” Espinosa says. “I invited Joel to come to our lab in Colorado to spend a few months learning how to work with human cancer cells.”
What they found is that the role of TIP60 is conserved in human colorectal cancer cells, which require HIF1A, CDK8 and TIP60 to form small tumors in the lab.
“By depleting CDK8 and TIP60 in colorectal cancer cells, we shut down more than 60 percent of the cellular activity of HIF1A, and this suffices to block their tumor-initiating ability,” Galbraith says.
As with much basic research, Perez-Perri’s stay in the Espinosa lab generated as many questions as answers, many having to do with molecular mechanisms by which TIP60 promotes HIF1A activity. This line of study was pursued in the Espinosa lab by graduate student Veronica Dengler. Dengler’s work showed that HIF1A and TIP60 work together to turn on many genes inside the cell nucleus, including key genes required for the cellular adaptation to hypoxia. In collaboration with scientists from Madison, WI, led by Dr. Danette Daniels at the biotech Promega, the team demonstrated that HIF1A and TIP60 interact physically with each other inside cells.
“What I love about this project is that it illustrates the power of collaboration and training efforts. In order to make these important discoveries, we assembled a team of scientists from Buenos Aires, Colorado and Wisconsin, in academia and industry, to work with two graduate students located 6,000 miles apart,” Espinosa says.
“This study absolutely demonstrates the importance of basic research,” Galbraith says. “Here we had something in a fruit fly that didn’t have any obvious connection to cancer and it turns out to be an important player in one of the most critical networks of cancer survival signaling.”
The researchers hope their work will stimulate additional interest in exploring the function and possible inhibition of TIP60 and CDK8 in the context of cancer.
###
Media Contact
Garth Sundem
[email protected]
@CUAnschutz
http://www.ucdenver.edu
The post Summer session fruit fly data leads to promising new target in colorectal cancer appeared first on Scienmag.