A study conducted by researchers in Brazil, Australia, Austria and the United States has made significant discoveries about a type of pediatric cancer with no approved drugs for treatment and with a low survival rate. The findings, described in an article published in the journal Neuro-Oncology, pave the way for a search for more specific therapies.
Credit: Taciani Magalhães
A study conducted by researchers in Brazil, Australia, Austria and the United States has made significant discoveries about a type of pediatric cancer with no approved drugs for treatment and with a low survival rate. The findings, described in an article published in the journal Neuro-Oncology, pave the way for a search for more specific therapies.
“Ependymomas are central nervous system tumors of various kinds that can basically be treated only by surgical removal and radiation therapy. Our study focused on supratentorial ependymoma with fusion of the genes C11orf95 and RELA [ST-RELA], a sub-group frequent in children. It’s aggressive, with a poor prognosis and no specific treatment,” said Taciani de Almeida Magalhães, first author of the article. The study was conducted during her PhD research at the University of São Paulo’s Ribeirão Preto Medical School (FMRP-USP) in Brazil, with FAPESP’s support.
The study was part of a Thematic Project led by Luiz Gonzaga Tone, a professor at FMRP-USP. Tone is Magalhães’s thesis advisor and penultimate author of the article.
Ependymoma is the third most common form of childhood brain and spine tumor, occurring mostly in infants and young children. It begins in the ependymal cells lining the hollow cavities within the brain (ventricles) that are filled with cerebrospinal fluid. Supratentorial refers to the upper part of the brain. Supratentorial ependymoma mainly affects children aged about 8 at the time of diagnosis. The five-year survival rate is about 30%, especially where complete surgical removal of the tumor is impossible. Radiation therapy can cause severe cognitive and motor complications.
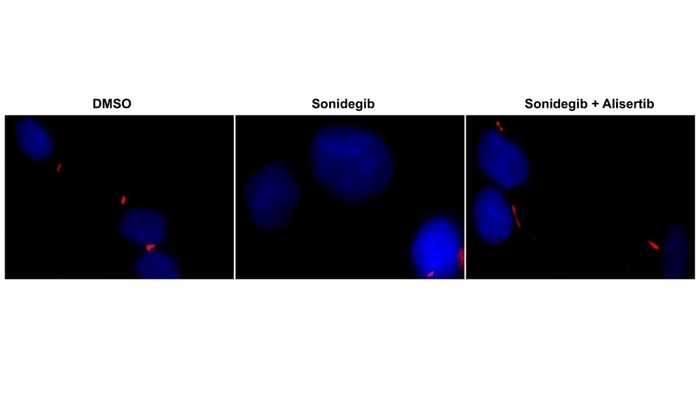
Using an array of advanced techniques, the researchers discovered that the so-called Hedgehog signaling pathway (Hh) is highly activated in this type of tumor. They treated tumors in the laboratory with Sonidegib, an Hh inhibitor currently undergoing clinical trials as a drug for other central nervous system tumors.
Analysis of the treated tumors showed loss of primary cilia, making them resistant to the drug. Primary cilia are organelles consisting of microtubules that extrude from the cell membrane into the interstitial space and communicate with the extracellular environment. They are essential to neurological development.
The researchers discovered that primary cilia formation was regulated by a specific protein called AURKA. This protein is present in other tumors and had previously been inhibited using Alisertib in clinical trials. They therefore treated the tumors with Alisertib as well as Sonidegib. The primary cilia were no longer lost and Sonidegib was able to act, leading to the death of tumor cells without any damage to healthy cells.
With the combination of drugs working well on the in vitro model, they next tested it on animals, in collaboration with a research group in Australia. To their surprise, the survival rate for mice with ependymoma treated with the combination did not rise compared with untreated mice used as controls.
The researchers believe the blood-brain barrier may have prevented the drugs from reaching the tumors. “Other studies have shown that inhibitors of AURKA, the protein that promotes primary cilia loss, didn’t reach the brain. This is a possible explanation for the failure of our treatment in animals,” said Magalhães, who is currently on a postdoctoral internship at Harvard Medical School in the United States. Previously she had conducted part of her PhD research at the same institution with a scholarship from FAPESP.
Alternatives
The researchers are now looking for other drugs with the same action that can penetrate the blood-brain barrier, potentially leading to treatment for the disease for the first time. “Although the combination was not successful in our animal model, we now understand the tumor’s molecular mechanisms and have a route to follow that was previously unknown,” Magalhães said.
For Elvis Terci Valera, a professor in FMRP-USP’s child health program and last author of the article, the discoveries open up a prospect of clinical studies using a more advanced generation of Hh and AURKA inhibitors capable of penetrating the central nervous system.
“Another strategy would be to apply these more modern drugs directly to the cerebrospinal fluid produced by ependymal cells in the ventricles of the brain, and to the spinal marrow. Options like this could be assessed as a way of reversing resistance to treatment,” Valera said.
About São Paulo Research Foundation (FAPESP)
The São Paulo Research Foundation (FAPESP) is a public institution with the mission of supporting scientific research in all fields of knowledge by awarding scholarships, fellowships and grants to investigators linked with higher education and research institutions in the State of São Paulo, Brazil. FAPESP is aware that the very best research can only be done by working with the best researchers internationally. Therefore, it has established partnerships with funding agencies, higher education, private companies, and research organizations in other countries known for the quality of their research and has been encouraging scientists funded by its grants to further develop their international collaboration. You can learn more about FAPESP at www.fapesp.br/en and visit FAPESP news agency at www.agencia.fapesp.br/en to keep updated with the latest scientific breakthroughs FAPESP helps achieve through its many programs, awards and research centers. You may also subscribe to FAPESP news agency at http://agencia.fapesp.br/subscribe
Journal
Neuro-Oncology
DOI
10.1093/neuonc/noac147
Article Title
Activation of Hedgehog signaling by the oncogenic RELA fusion reveals a primary cilia-dependent vulnerability in supratentorial ependymoma
Article Publication Date
31-May-2022



