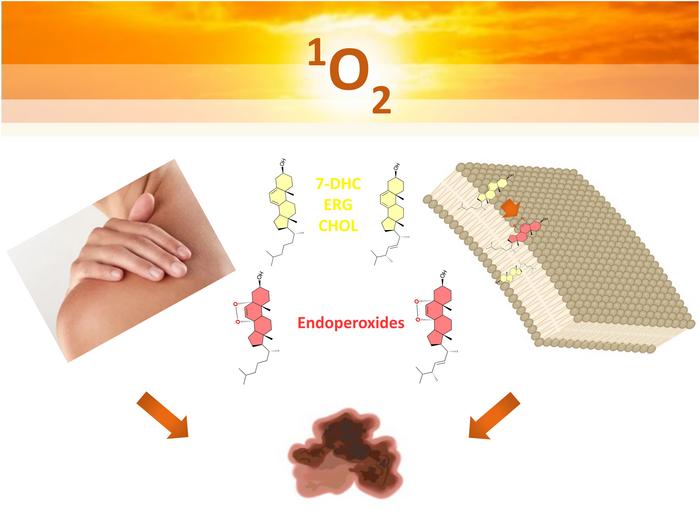
Photobiological processes underpin both the risks associated with and the therapeutic potential of light exposure. Naturally occurring photosensitizing molecules within the skin can absorb both ultraviolet and visible light, transforming photon energy into chemically reactive species. Among them, reactive oxygen species (ROS) play a pivotal role. They include free radicals and singlet oxygen, highly reactive oxidants capable of inflicting molecular damage to cellular structures such as membrane lipids and proteins. The dual nature of these reactions presents a unique paradox: while photo-oxidation can initiate carcinogenic pathways, these same mechanisms can be co-opted in photodynamic therapy (PDT) to selectively eradicate tumor cells.
Understanding the mechanistic underpinnings of how these photo-oxidation processes affect cell membranes was central to this study. Cell membranes are complex, fluid structures primarily composed of phospholipid bilayers interspersed with sterols such as cholesterol and its precursors. These sterols influence membrane fluidity, permeability, and resilience against oxidative assaults. Photo-oxidation reactions proceed by two fundamental pathways classified as type I and type II mechanisms. Type I involves the generation of radical species, such as superoxide anions and hydroperoxyl radicals, whereas type II reactions produce singlet molecular oxygen, a highly reactive form of oxygen capable of directly oxidizing membrane lipids.
Intriguingly, the study revealed that the protective role of sterols varies with the oxidative mechanism at play. Ergosterol and 7-DHC displayed superior membrane protection under the radical-mediated type I oxidation, while cholesterol conferred more robust protection during singlet oxygen-mediated type II processes. Cholesterol, known for its organizing role within membranes, appeared to limit singlet oxygen accessibility to vulnerable unsaturated lipids, thus acting as an intrinsic antioxidant within this oxidative context. These differential effects underscore the sophisticated balance between membrane composition and susceptibility to photo-oxidative damage.
While sterols serve antioxidant functions by shielding membranes, their oxidation inevitably yields a spectrum of products that can compromise membrane integrity. The formation of endoperoxides from ergosterol and 7-DHC was determined to be the most stable and biologically relevant outcome of these oxidation reactions. Prior research, including studies published in high-impact journals like Nature, has demonstrated that 7-DHC can act as an antioxidant, mitigating ferroptosis—an iron-dependent form of cell death driven by lipid peroxidation. However, this protective activity comes at the cost of sterol oxidation and generation of bioactive products, creating a complex interplay between cell survival and death.
Ergosterol, a sterol primarily found in yeast but structurally similar to 7-DHC, has been less studied in the context of oxidative membrane damage. The Redoxoma team addressed this gap by systematically examining the oxidative behavior of ergosterol compared to mammalian sterols. Their findings helped clarify previously conflicting reports on ergosterol’s role, confirming that its oxidation leads to endoperoxide formation with significant implications for membrane dynamics and melanoma cell susceptibility.
The São Paulo Research Foundation (FAPESP) played a pivotal funding role in supporting this cutting-edge research. As Brazil’s leading public institution dedicated to advancing all scientific fields, FAPESP emphasizes fostering international collaborations and innovation to elevate research quality. This study is emblematic of their mission to address global health challenges while bolstering scientific excellence within São Paulo and beyond.
Collectively, these findings illuminate a nuanced landscape where lipid oxidation and membrane biochemistry intersect with clinical oncology. By leveraging detailed mechanistic understanding of sterol photo-oxidation and its consequences in melanoma, researchers are paving the way for novel photodynamic therapeutic approaches. This line of inquiry not only accentuates the intricate balance of oxidative processes in cellular life and death but also demonstrates the promise of redox biochemistry in crafting next-generation cancer treatments with improved specificity and reduced side effects.
Subject of Research: Redox biology of sterol oxidation and photodynamic therapy in melanoma cells
Article Title: Comparative study of ergosterol and 7-dehydrocholesterol and their endoperoxides: Generation, identification, and impact in phospholipid membranes and melanoma cells
News Publication Date: 21-Jan-2025
Web References:
https://bv.fapesp.br/en/auxilios/58576
https://redoxoma.iq.usp.br/?hl=en
https://onlinelibrary.wiley.com/doi/10.1111/php.14059
References:
Miyamoto, S., Nishitani Yukuyama, M., et al. (2025). Comparative study of ergosterol and 7-dehydrocholesterol and their endoperoxides: Generation, identification, and impact in phospholipid membranes and melanoma cells. Photochemistry and Photobiology. DOI: 10.1111/php.14059
Image Credits: Redoxoma
Keywords: Melanoma cells, Cell therapies, Sterols, Ultraviolet radiation, Redox reactions, Skin cells
Tags: cellular damage from oxidative compoundseffects of UV radiation on skin cancerenvironmental risk factors for melanomainnovative treatments for aggressive skin cancermechanisms of melanoma metastasismelanoma skin cancer researchoxidative stress in melanoma cellsphoto-oxidation and carcinogenesisphotodynamic therapy for melanomaphotosensitizing molecules in skinrole of reactive oxygen species in cancertherapeutic potential of light exposure