Rice University bioscientists develop chromosome-level detectors to track activity in cells
Credit: Segatori Research Group/Rice University
HOUSTON – (March 9, 2020) – A novel system to amplify gene expression signals could be a game-changer for scientists who study the regulatory processes in cells that are central to all life.
The Rice University lab of bioscientist Laura Segatori has developed a versatile gene signal amplifier that can do a better job of detecting the expression of target genes than current methods.
Ultimately, the researchers hope the two-module system will simplify the diagnosis of diseases like Alzheimer’s, diabetes and some cancers characterized by distinctive patterns of protein expression. They said it could also enable cell-based therapies by which diseased cells could make their own medicine at the point of need.
Their work is described in Nature Chemical Biology.
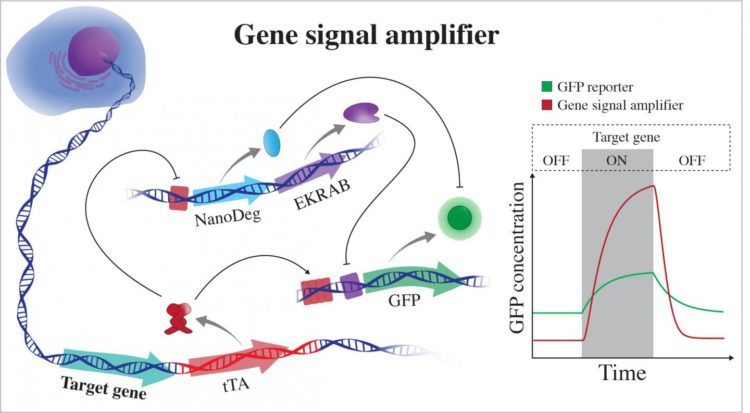
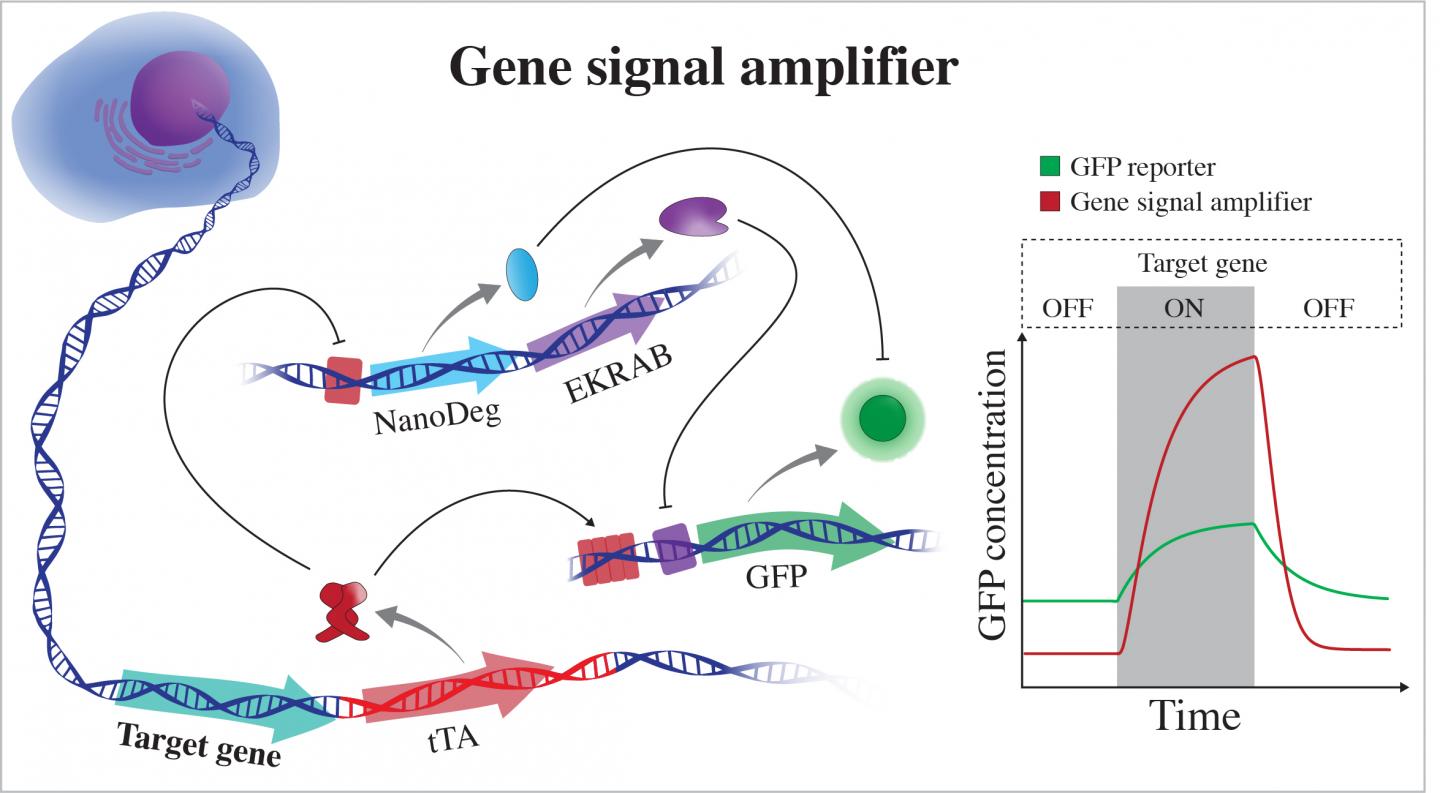
The first module is part of a string of synthetic genetic code added to the DNA of a mammalian cell via CRISPR-Cas9 editing. Once integrated adjacent to a target gene, the code enables a genetic circuit that monitors the gene and, whenever the gene produces a protein, the circuit also emits a green fluorescent protein (GFP). The circuit is designed to amplify the GFP signal and enable detection of very small changes in the target gene that are not always possible with current tools.
When the gene is inactive, the second module based on an antibody first found in camels stops production of the fluorescent protein and degrades any GFPs in the vicinity. The combination gives researchers a strong “on-off” signal that is also sensitive to the dynamics of expression of the target gene. When gene expression increases, the circuit activates expression of GFP and at the same time inhibits expression of negative regulators of GFP, such as the nanobody.
“Being able to monitor gene expression with high sensitivity is really important for a variety of biomedical applications,” Segatori said. “It’s important to have a detection system that is sensitive to even small changes in gene expression, which are often biologically relevant. It is also critical to a detection system that provides good dynamic resolution so we can follow gene expression dynamics, which are typically a key determinant of cell behavior.
“That’s what our gene signal amplifier essentially does,” she said. “We developed a genetic circuit that, first of all, we can link to any gene in the chromosome, thus generating a tool that recapitulates the chromosomal context with all the associated complexity of regulation. We don’t have any type of extrachromosomal reporters. This approach provides a sensitive way to monitor all the regulatory and epigenetic mechanisms that regulate gene expression.
“Then we developed a method to amplify the signal so we’re able to monitor really small changes in expression,” she said. “It’s very robust and stable and has high dynamic resolution.”
The system can be adapted to potentially monitor any cellular gene, Segatori said. “We can create multiplex reporter systems for monitoring group of genes that are relevant to the development of a certain disease or that provide a comprehensive readout for a certain signaling pathway or phenotype,” she said.
The team demonstrated the method on a variety of cells and generated a multiplex reporter to monitor markers associated with three signaling pathways that respond to stress in a mammalian cell’s endoplasmic reticulum. They found the circuit enhanced the fluorescent signal enough to detect even small changes in expression.
The second module, a NanoDeg circuit introduced by the Rice lab in 2017, is a post-translation control that gives the system its wide dynamic range, Segatori said. “Under basal conditions, the circuit expresses not only a transcriptional regulator that inhibits expression of GFP but also NanoDeg molecules that degrade any GFP present in the system, so the cell goes completely dark,” she said. “And we can tune the system to adapt it to detection of genes with different basal expression by using appropriate doses of inducers of the circuit components.”
Experiments confirmed that integrating the system into the cell’s chromosome does not affect the expression of target genes.
As part of the study, the lab also developed a mathematical model researchers can use to customize the amplifier platform to monitor any target gene and predict the optimal doses of small-molecule inducers used to regulate gene expression.
Segatori and her team are working to enhance the platform, primarily developed by Rice graduate student and lead author Carlos Origel Marmolejo, to treat disease.
“There is a great interest currently in developing cell therapies that are feedback-responsive,” Segatori said. “Our platform could allow production of therapeutics in response to detection of gene expression signatures relevant to a certain disease or environmental condition.”
###
Co-authors of the paper are graduate student Bhagyashree Bachhav and undergraduates Sahiti Patibandla and Alexander Yang. Segatori is an associate professor of bioengineering, chemical and biomolecular engineering and biosciences.
The National Science Foundation and the Welch Foundation supported the research.
Read the abstract at https:/
This news release can be found online at https:/
Follow Rice News and Media Relations via Twitter @RiceUNews.
Related materials:
Nanoscale platform aims to control protein levels: http://news.
Synthetic gene circuits pump up cell signals: http://news.
Segatori Research Group: http://segatori.
Department of BioSciences: http://biosciences.
Department of Bioengineering: https:/
Department of Chemical and Biomolecular Engineering: https:/
Images for download:
https:/
The gene signal amplifier developed by bioscientists at Rice University excels at detecting the expression of target genes and can also be used to detect potentially any cellular gene. The amplifier is linked to a cell’s chromosome and directly reports on the activity of a gene by expressing fluorescent proteins (GFP). When the gene is not active, the amplifier expresses negative regulators that quench GFP by operating at different hierarchical levels of cellular information flow. EKRAB is a transcriptional repressor and NanoDeg is a post-translational regulator. When the gene is active, tTA produces GFP and blocks expression of the negative regulators. (Credit: Segatori Research Group/Rice University)
https:/
Rice University researchers — including, from left, Sahiti Patibandla, Laura Segatori and Bhagyashree Bachhav — have developed a versatile gene signal amplifier that detects the expression of chromosomal genes. (Credit: Jeff Fitlow/Rice University)
Located on a 300-acre forested campus in Houston, Rice University is consistently ranked among the nation’s top 20 universities by U.S. News & World Report. Rice has highly respected schools of Architecture, Business, Continuing Studies, Engineering, Humanities, Music, Natural Sciences and Social Sciences and is home to the Baker Institute for Public Policy. With 3,962 undergraduates and 3,027 graduate students, Rice’s undergraduate student-to-faculty ratio is just under 6-to-1. Its residential college system builds close-knit communities and lifelong friendships, just one reason why Rice is ranked No. 1 for lots of race/class interaction and No. 4 for quality of life by the Princeton Review. Rice is also rated as a best value among private universities by Kiplinger’s Personal Finance.
Media Contacts:
Jeff Falk
713-348-6775
[email protected]
Mike Williams
713-348-6728
[email protected]
Media Contact
Mike Williams
[email protected]
713-348-6728
Original Source
https:/
Related Journal Article
http://dx.