Credit: Sharmila Samaroo/University of Michigan.
Because the sun doesn't always shine, solar utilities need a way to store extra charge for a rainy day. The same goes for wind power facilities, since the wind doesn't always blow. To take full advantage of renewable energy, electrical grids need large batteries that can store the power coming from wind and solar installations until it is needed. Some of the current technologies that are potentially very appealing for the electrical grid are inefficient and short-lived.
University of Utah and University of Michigan chemists, participating in the U.S. Department of Energy's Joint Center for Energy Storage Research, predict a better future for a type of battery for grid storage called redox flow batteries. Using a predictive model of molecules and their properties, the team has developed a charge-storing molecule around 1,000 times more stable than current compounds. Their results are reported today in the Journal of the American Chemical Society.
"Our first compound had a half-life of about eight-12 hours," says U chemist Matthew Sigman, referring to the time period in which half of the compound would decompose. "The compound that we predicted was stable on the order of months."
Not your ordinary battery
For a typical residential solar panel customer, electricity must be either used as it's generated, sold back to the electrical grid, or stored in batteries. Deep-cycle lead batteries or lithium ion batteries are already on the market, but each type presents challenges for use on the grid.
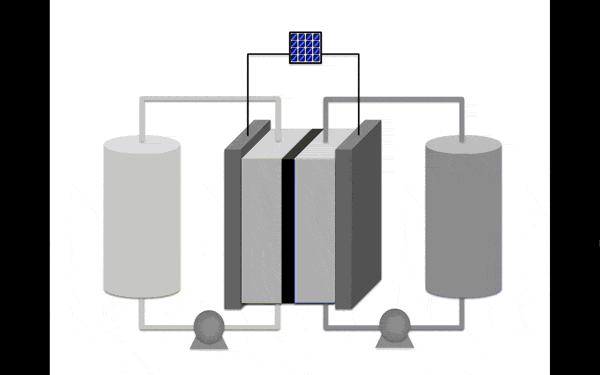
All batteries contain chemicals that store and release electrical charge. However, redox flow batteries aren't like the batteries in cars or cell phones. Redox flow batteries instead use two tanks to store energy, separated by a central set of inert electrodes. The tanks hold the solutions containing molecules or charged atoms, called anolytes and catholytes, that store and release charge as the solution "flows" past the electrodes, depending on whether electricity is being provided to the battery or extracted from it.
"If you want to increase the capacity, you just put more material in the tanks and it flows through the same cell," says University of Michigan chemist Melanie Sanford. "If you want to increase the rate of charge or discharge, you increase the number of cells."
Current redox flow batteries use solutions containing vanadium, a costly material that requires extra safety in handling because of its potential toxicity. Formulating the batteries is a chemical balancing act, since molecules that can store more charge tend to be less stable, losing charge and rapidly decomposing.
Molecular bumper cars
Sanford began collaborating with Sigman and U electrochemist Shelley Minteer through the U.S. Department of Energy's Joint Center for Energy Storage Research (JCESR), an Energy Innovation Hub dedicated to creating next-generation battery technologies. Sanford's lab developed and tested potential electrolyte molecules, and sought to use predictive technology to help design better battery compounds. Minteer contributed expertise in electrochemistry and Sigman employed a computational method, which uses the structural features of a molecule to predict its properties. A similar approach is widely used in drug development to predict the properties of candidate drugs.
The team's work found that a candidate compound decomposed when two molecules interacted with each other. "These molecules can't decompose if they can't come together," Sanford says. "You can tune the molecules to prevent them from coming together."
Tuning a key parameter of those molecules, a factor describing the height of a molecular component, essentially placed a bumper or deflector shield around the candidate molecule.
The most exciting anolyte reported in the paper is based on the organic molecule pyridinium. It contains no metals and is intended to be dissolved in an organic solvent, further enhancing its stability. Other compounds exhibited longer half-lives, but this anolyte provides the best combination of stability and redox potential, which is directly related to how much energy it can store.
Sharing skills to build batteries
Sigman, Minteer and Sanford are now working to identify a catholyte to pair with this and future molecules. Other engineering milestones lay ahead in the development of a new redox flow battery technology, but determining a framework for improving battery components is a key first step.
"It's a multipart challenge, but you can't do anything if you don't have stable molecules with low redox potentials," Sanford says. "You need to work from there."
The team attributes their success thus far to the application of this structure-function relationship toolset, typically used in the pharmaceutical industry, to battery design. "We bring the tools of chemists to a field that was traditionally the purview of engineers," Sanford says.
###
This release and an accompanying GIF can be found here.
Find the full study here.
Funding for the project was provided by the Joint Center for Energy Storage Research, a Department of Energy Innovation Hub supported by DOE's Office of Science.
The Joint Center for Energy Storage Research (JCESR), a DOE Energy Innovation Hub, is a major partnership that integrates researchers from many disciplines to overcome critical scientific and technical barriers and create new breakthrough energy storage technology. Led by the U.S. Department of Energy's Argonne National Laboratory, partners include national leaders in science and engineering from academia, the private sector, and national laboratories. Their combined expertise spans the full range of the technology-development pipeline from basic research to prototype development to product engineering to market delivery.
Media Contact
Paul Gabrielsen
[email protected]
801-505-8253
@uofunews
http://www.unews.utah.edu/
############
Story Source: Materials provided by Scienmag