Credit: Ozbolat laboratory, Penn State
Fixing traumatic injuries to the skin and bones of the face and skull is difficult because of the many layers of different types of tissues involved, but now, researchers have repaired such defects in a rat model using bioprinting during surgery, and their work may lead to faster and better methods of healing skin and bones.
“This work is clinically significant,” said Ibrahim T. Ozbolat, Hartz Family Career Development Associate Professor of Engineering Science and Mechanics, Biomedical Engineering and Neurosurgery, Penn State. “Dealing with composite defects, fixing hard and soft tissues at once, is difficult. And for the craniofacial area, the results have to be esthetically pleasing.”
Currently, fixing a hole in the skull involving both bone and soft tissue requires using bone from another part of the patient’s body or a cadaver. The bone must be covered by soft tissue with blood flow, also harvested from somewhere else, or the bone will die. Then surgeons need to repair the soft tissue and skin.
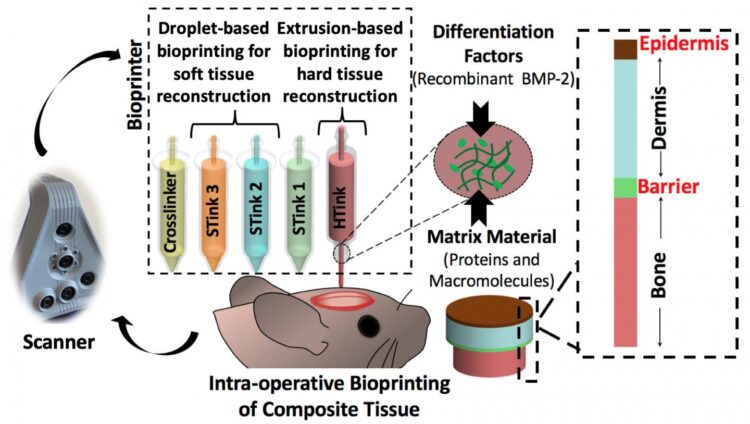
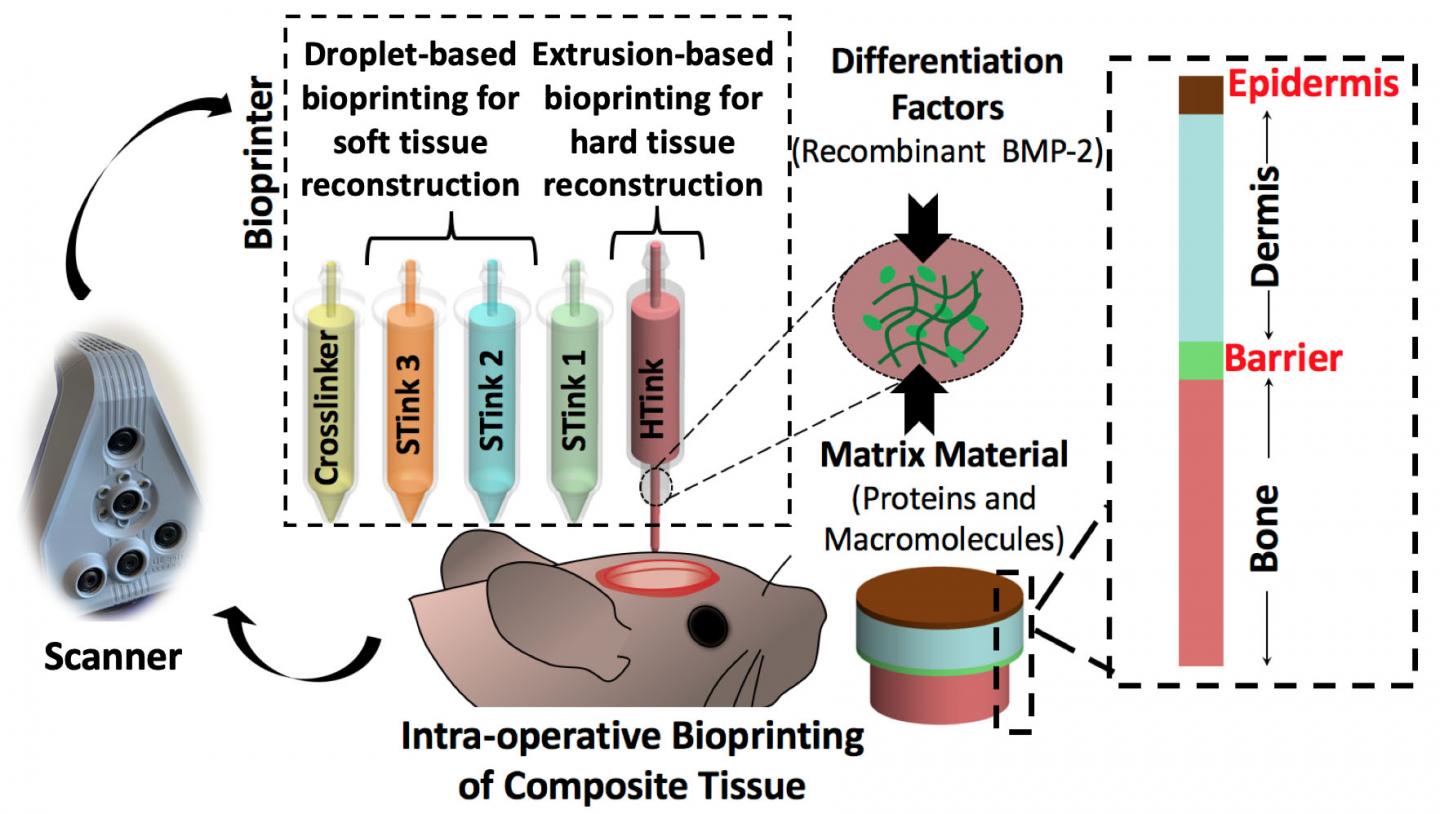
Ozbolat and his team used extrusion bioprinting and droplet bioprinting of mixtures of cells and carrier materials to print both bone and soft tissue. They report their results in Advanced Functional Materials.
“There is no surgical method for repairing soft and hard tissue at once,” said Ozbolat. “This is why we aimed to demonstrate a technology where we can reconstruct the whole defect — bone to epidermis — at once.”
The researchers attacked the problem of bone replacement first, beginning in the laboratory and moving to an animal model. They needed something that was printable and nontoxic and could repair a 5-millimeter hole in the skull. The “hard tissue ink” consisted of collagen, chitosan, nano-hydroxyapatite and other compounds and mesenchymal stem cells — multipotent cells found in bone marrow that create bone, cartilage and bone marrow fat.
The hard tissue ink extrudes at room temperature but heats up to body temperature when applied. This creates physical cross-linkage of the collagen and other portions of the ink without any chemical changes or the necessity of a crosslinker additive.
The researchers used droplet printing to create the soft tissue with thinner layers than the bone. They used collagen and fibrinogen in alternating layers with crosslinking and growth enhancing compounds. Each layer of skin including the epidermis and dermis differs, so the bioprinted soft tissue layers differed in composition.
Experiments repairing 6 mm holes in full thickness skin proved successful. Once the team understood skin and bone separately, they moved on to repairing both during the same surgical procedure.
“This approach was an extremely challenging process and we actually spent a lot of time finding the right material for bone, skin and the right bioprinting techniques,” said Ozbolat.
After careful imaging to determine the geometry of the defect, the researchers laid down the bone layer. They then deposited a barrier layer mimicking the periosteum, a heavily vascularized tissue layer that surrounds the bone on the skull.
“We needed the barrier to ensure that cells from the skin layers didn’t migrate into the bone area and begin to grow there,” said Ozbolat.
After laying down the barrier, the researchers printed the layers of dermis and then the epidermis.
“It took less than 5 minutes for the bioprinter to lay down the bone layer and soft tissue,” said Ozbolat.
The researchers performed more than 50 defect closures and achieved 100% closure of soft tissue in four weeks. The closure rate for bone was 80% in six weeks, but Ozbolat noted that even with harvested bone replacement, bone closure usually does not reach 100% in six weeks.
According to Ozbolat, blood flow to the bone is especially important and inclusion of vascularizing compounds is a next step.
The researchers also want to translate this research to human applications and are continuing to work with neurosurgeons, craniomaxillofacial surgeons and plastic surgeons at Penn State Hershey Medical Center. They operate a larger bioprinting device on larger animals.
###
Other researchers from Penn State working on this project include Kazim K. Moncal, recent doctoral degree recipient; Hemanth Gudapati, recent doctoral degree recipient; and Youngnam Kang, postdoctoral fellow; all in engineering science and mechanics. Kevin P. Godzik, recent bachelor’s recipient in biomedical engineering; Jason Z. Moore, associate professor in mechanical and biomedical engineering; and David F. Pepley, doctoral degree recipiant in mechanical engineering. At Penn State Hershey Medical Center were Hwa Bok Wee, research associate, and Gregory S. Lewis, assistant professor, Orthopedics and Rehabilitation; Elias Rizk, associate professor of neurosurgery, Thomas D. Samson, associate professor of plastic surgery, and Dino J. Ravnic, assistant professor of plastic surgery; all in the College of Medicine.
Others were Dong N. Heo, former postdoctoral fellow and now research professor of dental materials, Kyung Hee University, Seoul, Republic of Korea; Veli Ozbolat, former postdoctoral fellow and now assistant professor, Cukorova University, Adana, Turkey; and Ryan R. Driskell, assistant professor of molecular biosciences, Washington State University.
The National Institute of Dental and Craniofacial Research, National Science Foundation, Osteology Foundation and International Team for Implantology supported this work.
Media Contact
A’ndrea Elyse Messer
[email protected]
Related Journal Article
http://dx.