Research teams comprising distinguished scientists from Pohang University of Science and Technology (POSTECH) and Seoul National University Hospital have made remarkable advancements in the field of neurobiology by successfully developing a three-dimensional (3D) model that accurately replicates the Blood-Brain Barrier (BBB). This groundbreaking research, recently published in the esteemed journal Biomaterials Research, holds the potential to dramatically enhance our understanding of neurodegenerative diseases, including Alzheimer’s, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS). These conditions are characterized by the gradual degradation of brain and nervous system functionalities primarily driven by aging. Chronic neuroinflammation is a significant contributor to these pathologies, and the novel BBB model plays a crucial role in elucidating the complex interactions that occur between cerebral blood vessels and neural cells.
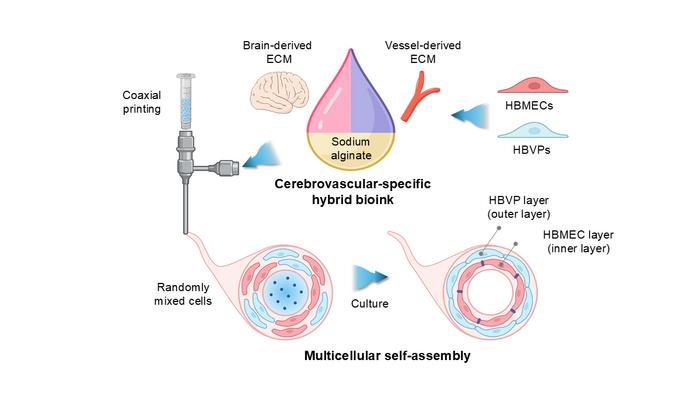
Despite the critical nature of the BBB in maintaining central nervous system homeostasis, existing models have struggled to accurately replicate its intricate organization. Traditional two-dimensional (2D) models have not been able to emulate the multifaceted three-dimensional architecture of cerebral blood vessels effectively. Researchers have consistently faced considerable obstacles in utilizing these inadequate models for drug development and understanding the pathophysiology of neurodegenerative diseases. This new study addresses these challenges by introducing a highly sophisticated cerebrovascular-specific bioink derived from decellularized extracellular matrix (CBVdECM). This bioink, sourced from porcine brain and blood vessels, has allowed scientists to leverage 3D bioprinting technology to construct an anatomically precise tubular vascular model reflective of the human BBB’s structural and functional properties.
One of the television stars of this innovative model is its inherent capacity for spontaneous self-assembly into a dual-layered vascular structure without requiring any external stimuli. When researchers incorporated human brain microvascular endothelial cells (HBMEC) and human brain vascular pericytes (HBVP) into the CBVdECM bioink, they observed that the endothelial cells naturally organized themselves into an inner vascular wall. At the same time, pericytes constructed a surrounding layer, resulting in a remarkably realistic two-layered structure akin to actual cerebral blood vessels.
The research team further advanced their experimental framework by successfully replicating the organization of tight junction proteins typically absent in conventional 2D BBB models. These proteins are essential for the formation of the selective permeability characteristic of the BBB, thus allowing researchers to glean new insights into how these junctions contribute to the barrier’s function in disease contexts. Furthermore, they meticulously examined the BBB’s permeability along with its inflammatory responses when subjected to inflammation-inducing substances such as TNF-α and IL-1β. This capability to model neuroinflammatory mechanisms precisely represents a significant advancement in our understanding of BBB dysfunction and its relationship with neurodegenerative diseases.
The potential implications of this research are profound, offering a new platform for investigating the underlying pathological mechanisms of neuroinflammatory diseases while simultaneously serving as a testbed for the development of innovative therapeutic strategies. Professor Sun Ha Paek, one of the lead researchers from Seoul National University Hospital, remarked on the importance of this study, emphasizing its utility in revealing the intricate dynamics of neuroinflammation. Additionally, Professor Jinah Jang from POSTECH mentioned plans to incorporate various other cell types into their models—such as glial cells, neurons, and immune cells in order to further refine methods for quantifying inflammatory responses and permeability.
As the research progresses, the ultimate goal is to expand these models into patient-specific disease contexts, potentially revolutionizing the landscape for studying neuroinflammatory diseases within personalized medicine frameworks. The collaborative effort exemplifies how interdisciplinary research combining engineering, life sciences, and medicine can yield breakthrough advancements, promising a future where tailored therapeutic interventions can be based on individual patient profiles.
Such technological innovations in the field of bioengineering underscore the significance of 3D bioprinting as a transformative tool in biomedical research. By allowing researchers to fabricate more accurate and representative biological models, 3D bioprinting paves the way toward creating complex tissue structures necessary for a myriad of applications ranging from drug testing to regenerative medicine.
Considering the gravity of neurodegenerative diseases and their impact on global health, this research is not just an academic exercise; it possesses the potential to ignite considerable advancements in how these diseases are understood and treated. The multi-faceted approach adopted by the researchers significantly enhances our capacity to explore and test novel therapeutic strategies aimed at bolstering brain health and function.
Moreover, this remarkable study highlights the urgent need for more sophisticated in vitro models to deduce the cellular and molecular mechanisms that drive the onset and progression of pathologies associated with BBB disruption. The development of an accurate and functional BBB model could be a game-changer for pharmaceutical companies seeking to develop effective treatments for neurodegenerative diseases, which have long been considered some of the most difficult conditions to target therapeutically.
As investigators continue to delve into this innovative realm of research, the impetus towards understanding how the BBB can be preserved or restored in neurodegenerative diseases gains familiarity and urgency. With the support from governmental and institutional initiatives, such as the Ministry of Trade, Industry & Energy and the National Research Foundation of Korea, researchers are equipped with the resources necessary to extend their investigations into uncharted territories, illuminating new pathways for therapeutic intervention.
Ultimately, this multidisciplinary research stands as a beacon of hope, suggesting that through the convergence of engineering and life sciences, there exists a tangible opportunity to revolutionize the way we study and treat some of the most challenging ailments afflicting humanity. As knowledge surrounding the complexities of the BBB expands, we might be approaching a new frontier in the field of neurobiology, one that offers unprecedented potential to ameliorate neurodegenerative conditions that plague millions worldwide.
In conclusion, while the research led by POSTECH and Seoul National University Hospital represents only the initial strides in a much larger journey, its implications are significant. The advent of a more sophisticated BBB model could serve as the critical first step in finding effective, innovative treatments for widespread neurodegenerative diseases that currently lack satisfactory therapeutic options.
Subject of Research: Blood-Brain Barrier Models and Neurodegenerative Diseases
Article Title: Cerebrovascular-Specific Extracellular Matrix Bioink Promotes Blood–Brain Barrier Properties
News Publication Date: 5-Dec-2024
Web References:
References:
Image Credits: Credit: POSTECH
Keywords
3D Bioprinting, Blood-Brain Barrier, Neurodegenerative Diseases, Chronic Neuroinflammation, Extracellular Matrix Bioink, Cerebral Blood Vessels, Microvascular Endothelial Cells, Neuroinflammatory Mechanisms, Therapeutic Strategies, In Vitro Models, Personalized Medicine, Biomedical Engineering.
Tags: 3D model for drug developmentadvanced neurobiology researchALS and Parkinson’s disease studiesAlzheimer’s disease researchbiomaterials in neuroscienceBlood-Brain Barrier modelcerebral blood vessel interactionschronic neurodegeneration mechanismsinnovative biomedical engineering solutionsneuroinflammation and neurodegenerative diseasesPOSTECH and Seoul National University collaborationself-assembling blood vessels