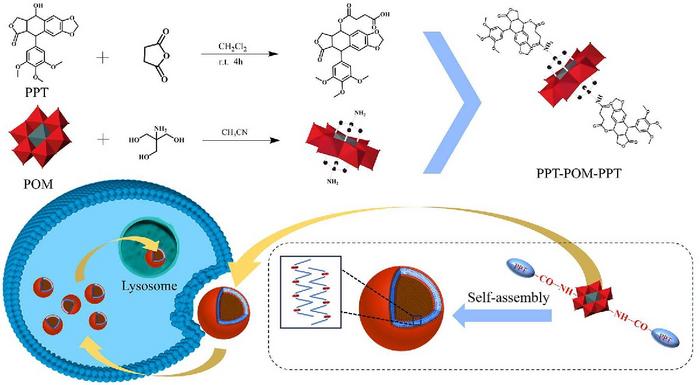
In a groundbreaking study, researchers have developed a new amphiphilic drug molecule known as PPT-POM-PPT that combines the cancer-fighting properties of podophyllotoxin (PPT) with the unique structure of polyoxometalates (POMs). This innovative molecule is designed to address significant limitations associated with traditional POM therapy, including poor cell membrane permeability due to its hydrophilic nature. The researchers have found that by chemically linking PPT to POMs, they can create a compound that self-assembles into stable nanoscale vesicles. These vesicles exhibit not only enhanced drug delivery capabilities but also potent anticancer activity.
The amphiphilic nature of PPT-POM-PPT is critical to its success in cancer therapy. By integrating the hydrophobic characteristics of PPT with the hydrophilic structures of POMs, the molecule naturally forms vesicles in an aqueous environment. This self-assembly is not just a fascinating chemical reaction; it is a sophisticated solution to one of the biggest challenges in drug delivery — ensuring that therapeutic agents effectively reach cancer cells while minimizing adverse side effects onnormal cells. The research team utilized techniques such as transmission electron microscopy and zeta potential analysis to confirm the successful formation of these hollow vesicles, which are distinguished by their negative charge and unique morphology.
The study revealed that the PPT-POM-PPT vesicles demonstrated remarkable stability and biocompatibility, which are crucial for any drug intended for therapeutic use. In vitro cytotoxicity assays indicated that these vesicles exhibit significant anticancer activity against various tumor cell lines, including H1299, A549, and Hep-G2 cells, while presenting lower toxicity levels towards HEK293 normal cells. This selective targeting capability is a promising sign for the future of cancer therapies, as it suggests that PPT-POM-PPT can inhibit tumor growth without adversely affecting healthy cells.
Furthermore, the research team highlighted the synergy between the two active components of the PPT-POM-PPT compound. By combining the anti-tumor effects of PPT with the inherent properties of POMs, this novel drug molecule capitalizes on their complementary strengths, leading to a stronger overall therapeutic effect. Such a dual mechanism of action could pave the way for more effective treatment options, addressing the urgent need for innovative cancer therapies that can outperform existing options.
One of the most compelling aspects of this research is the potential for simplified drug formulation. The team noted that the vesicular structures can deliver PPT-POM-PPT effectively without the need for additional carrier materials. This means that future formulations could be streamlined, reducing production complexities and costs while enhancing drug stability and patient compliance. Moreover, by leveraging the innate properties of POMs that promote vesicle formation, this approach minimizes potential risks associated with traditional drug delivery systems reliant on complex carriers.
The implications of this research extend beyond just chemical innovation. It represents a significant leap towards targeted cancer therapies that employ intelligent design principles. As the study was published in the esteemed journal Polyoxometalates, it stands to gain recognition within the scientific community, potentially inspiring further research on amphiphilic drug molecules and their applicability in various therapeutic contexts.
The study’s senior authors, Dejin Zang and Teng Liu, underscored the importance of this work in their statements. They described how the ability of PPT-POM-PPT to form vesicles that maintain their integrity and functionality in biological environments could lead to more advanced clinical applications. Their ongoing research efforts continue to focus on optimizing these compounds for maximum efficacy in real-world medical settings.
Additionally, funding for this research was supported by various prestigious organizations, including the Natural Science Foundation of China and the Young Scientist Development Foundation of Shandong First Medical University. Such backing is a testament to the importance of this line of inquiry and its potential long-term impact on cancer treatment strategies. The collaboration of multiple researchers across various institutions has fostered a rich academic environment that promotes groundbreaking discoveries like those seen with PPT-POM-PPT.
As this innovative technology advances, the future of cancer treatment could see a paradigm shift, with self-assembled drug molecules becoming standard practice in therapeutic protocols. The intricate design and functional capabilities of PPT-POM-PPT demonstrate how interdisciplinary research can yield powerful new tools against cancer. This amalgamation of chemistry and medical science could lead to protocols that offer higher success rates in treating patients while reducing collateral damage to healthy tissues, changing the landscape of oncology.
Moreover, the promise shown by the PPT-POM-PPT molecule may inspire further exploration into the frontiers of drug design, especially for conditions that require targeted delivery, such as autoimmune diseases or infectious disorders. As we march towards the future of medicine, innovative approaches to drug delivery—like the formulation of self-assembling vesicles—may unlock solutions to challenges that have long plagued the pharmaceutical industry.
In conclusion, the advancement of PPT-POM-PPT as a new potential candidate for cancer therapy promises to harness the power of simplicity, harmony between chemical properties, and innovative design. The research collectively embodies a key turning point in the ongoing battle against cancer. As more studies validate and expand on these findings, scientists and medical professionals may find themselves equipped with more effective weapons in the war on cancer, ensuring a healthier future for patients worldwide.
Subject of Research: Development of an amphiphilic drug molecule for antitumor therapy
Article Title: Self-assembled vesicles containing podophyllotoxin covalently modified with polyoxometalates for antitumor therapy
News Publication Date: 19-Mar-2025
Web References: Polyoxometalates Journal
References: DOI: 10.26599/POM.2025.9140085
Image Credits: Credit: Polyoxometalates, Tsinghua University Press
Keywords: amphiphilic drug molecule, self-assembly, podophyllotoxin, polyoxometalates, cancer therapy, drug delivery, cytotoxicity, vesicles, biocompatibility, cancer treatment, dual mechanism, innovative drug design.
Tags: amphiphilic drug molecules for cancer treatmentchemical bonding in drug designenhanced antitumor treatment strategiesimproved cell membrane permeabilityinnovative cancer therapeutics developmentnanoscale vesicles for drug deliverypodophyllotoxin anticancer propertiespolyoxometalates in cancer therapyself-assembled drug delivery systemstransmission electron microscopy in drug researchvesicle stability in aqueous environmentszeta potential analysis in nanomedicine