A research team led by Prof. CHEN Di from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences along with other collaborators have revealed the molecular mechanisms behind bone loss caused by chronic liver injury.
Credit: SIAT
A research team led by Prof. CHEN Di from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences along with other collaborators have revealed the molecular mechanisms behind bone loss caused by chronic liver injury.
Their study was published in Cell Metabolism on March 1.
Hepatic osteodystrophy disease (HOD) is a kind of metabolic bone disease that occurs in patients with chronic liver disorder. It mainly manifests as bone loss, bone density reduction, and destruction of bone structure.
As the body’s metabolic center, the liver plays an important role in the maintenance of tissue homeostasis and a large number of hepatic cytokines regulate peripheral organs, including bones, through the circulatory system.
This mutual regulation between liver and bone is called the liver-bone axis. External stimuli, such as viruses, alcohol, and drugs, may cause chronic liver damage, which subsequently affects bone metabolism through the liver-bone axis, resulting in an increased risk of osteoporosis and fragile fractures.
The fractures caused by HOD disease make bone reconstruction difficult, and severely affect disease prognosis and HOD patients’ quality of life. Thus, it is important to elucidate the mechanisms of HOD.
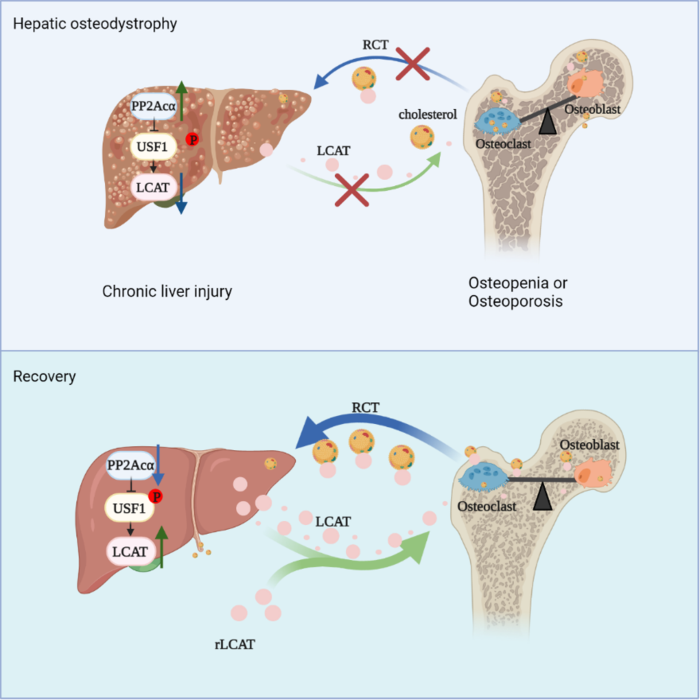
In patients with HOD and mouse models of HOD, the research team found high expression of PP2Acα. “Conditional knockout of PP2Acα in the liver of HOD mice helps the recovery of liver function and alleviates bone loss,” said Prof. CHEN.
Through proteomics analysis, the research team screened and identified the hepatic factor LCAT, the liver-bone axis regulator. As a cholesterol transferring enzyme, LCAT is able to transfer cholesterol from peripheral tissues to the liver, a process known as reverse cholesterol transport (RCT).
“LCAT mediates bone metabolism by maintaining appropriate intracellular cholesterol levels and improves liver function by reversing cholesterol transport from bone tissues to the liver,” said Dr. LU Ke, first author of the study.
RCT plays an important role in maintaining liver-bone homeostasis. Appropriate intracellular cholesterol levels can promote osteoblast function and inhibit osteoclast differentiation.
In patients with HOD and mouse models of HOD, the researchers found that PP2Acα down-regulated LCAT expression in HOD.
This study shows that imbalance of the liver-bone axis accelerates the progression of HOD caused by chronic liver injury. It also provides a potential target for the development of therapeutic drugs to treat hepatic bone disease.
Journal
Cell Metabolism
Article Title
Defects in a liver-bone axis contribute to hepatic osteodystrophy disease progression
Article Publication Date
1-Mar-2022




