Sleep is a critical period for memory consolidation, and most people don’t get enough. Research has shown that even brief periods of sleep deprivation can lead to deficits in memory formation. In a new study, published in the Journal of Neuroscience, a team led by scientists from the University of Pennsylvania found that a particular set of cells in a small region of the brain are responsible for memory problems after sleep loss. By selectively increasing levels of a signaling molecule in these cells, the researchers prevented mice from having memory deficits.
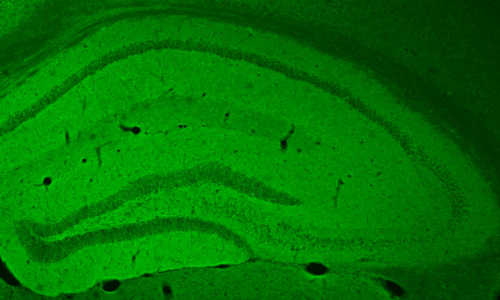
The hippocampus of a mouse in the study glows green, showing where excitatory neurons took up a cAMP-triggering receptor. Photo Credit: Image courtesy of University of Pennsylvania
Robbert Havekes was the lead author on the study. He is a research associate in the lab of Ted Abel, the study’s senior author and Brush Family Professor of Biology in Penn’s School of Arts & Sciences. Coauthors from the Abel lab included Jennifer C. Tudor and Sarah L. Ferri. They collaborated with Arnd Baumann of Forschungszentrum Jülich, Germany, and Vibeke M. Bruinenberg and Peter Meerlo of the University of Groningen, The Netherlands.
In 2009, a group from Abel’s lab published a study in Nature that identified the cyclic AMP, or cAMP, signaling pathway as playing a role in sleep-loss-associated memory problems. Whereas depriving mice of sleep impaired their spatial memory, restoring levels of cAMP in their brain prevented this effect.
“The challenge following this important study,” Abel said, “was to determine if the impact of sleep deprivation was mediated by particular regions of the brain and particular neural circuits. We suspected that the hippocampus, the brain region that mediates spatial navigation and contextual memory, was critical.”
In the current work, they set out to answer these questions. They targeted excitatory neurons because of their importance in transmitting signals in the brain and the fact that their functioning relies on cAMP signaling. The limitation of previous studies was that they lacked a way to increase cAMP in just one area of the brain in a cell-type specific fashion. Havekes, Abel and colleagues devised a way of doing this that they term a “pharmacogenetic” approach, blending genetic modification and drug administration.
They engineered a non-pathogenic virus to harbor the gene encoding the receptor for the protein octopamine, which triggers cAMP pathway activation in fruit flies but is not naturally found in the brains of mice. The researchers injected this virus into the hippocampus of mice so that the excitatory neurons in that region alone would express the octopamine receptor.
“It sounds weird. Why would you put a receptor there that is never going to be activated?” Havekes said. “The trick is, you follow that up by giving mice the ligand of the receptor, which is octopamine, and that will activate the receptors only where they are present.”
The team confirmed that only the excitatory hippocampal neurons expressed the receptor and that they could selectively increase cAMP levels in only these cells by giving the mice a systemic injection of octopamine.
“This way, we could manipulate the cAMP pathways that we previously saw being affected by sleep deprivation but selectively in specific neural circuits in the brain,” Havekes says.
With this pharmacogenetic tool in hand, Havekes, Abel and colleagues began the sleep deprivation tests with the mice expressing the octopamine receptor in their hippocampus. First the researchers trained mice in a spatial memory task. They put them in a box that had three different objects, each in a distinct location.
Then, because previous research had shown that cAMP signaling contributes to hippocampus-dependent memory consolidation in two time windows — first directly after training and again three to four hours after training — the researchers gave mice in the experimental groups injections of octopamine in both of these windows to boost cAMP levels.
Mice receiving the cAMP boost were divided into two groups: One was left to sleep undisturbed, while the other was sleep-deprived for five hours by gently tapping their cage or rearranging their bedding.
One full day after the initial training, all of the mice were tested again. This time, there was a twist: one of the objects originally in the box had been moved to a new location.
“If the mice had learned and remembered the location of the objects during their training, then they would realize, okay, this is the object that has moved, and they’ll spend more time exploring that particular object,” Havekes explained. “If they didn’t remember well, they would explore all the objects in a random fashion.”
The researchers found that the sleep-deprived mice that received the octopamine injections spent more time exploring the object that had moved, just as mice that had not been sleep deprived did. On the other hand, sleep-deprived mice that didn’t express the receptor explored all the objects at random, a sign that they had failed to remember the locations of the objects from their initial training as a result of the brief period of sleep deprivation.
“What we’ve shown is this memory loss due to sleep deprivation is really dependent on misregulation of cAMP signaling in the excitatory neurons of the hippocampus,” Havekes said.
As a next step, the group would like to explore what cAMP is doing to help consolidate memory. They would also like to investigate how other cell types in the brain, such as astrocytes, might be affected. And finally, while this study focused on the impact of a brief period of sleep deprivation, Havekes is curious to know how not getting enough sleep on a daily basis, as is more similar to human experiences, might be affecting memory.
“Thinking about people who do shift work or doctors who work long hours, if we can tackle the cognitive problems that result from sleep loss, that would be a great thing,” Havekes said.
“At least in the mouse using these sophisticated tools, we’re able to reverse the negative impact of sleep deprivation on cognition,” Abel said.
Story Source:
The above story is based on materials provided by University of Pennsylvania.