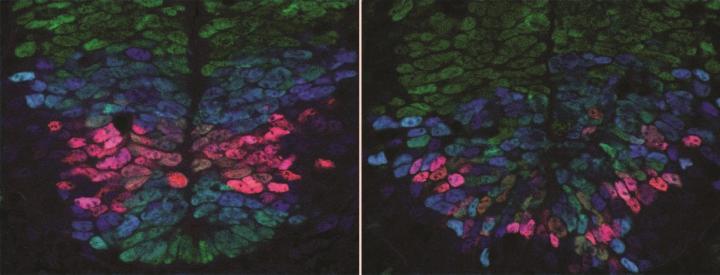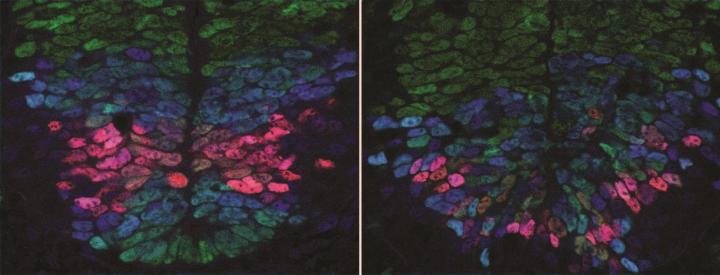
Credit: Anna Kicheva
Scientists have uncovered how nerve cells in the spinal cord are organised in precise patterns during embryo development – a finding that could give insight into regenerative medicine.
As embryos grow and develop they need the right cell types to end up in the right places inside forming organs. This is particularly important in the spinal cord where different nerve cell types must be accurately positioned so that circuits can assemble properly to control muscle movement. But until now the mechanism underlying nerve cell organisation in the spinal cord has remained poorly understood.
In a study published in Science, researchers at the Francis Crick Institute, the Institute of Science and Technology (Austria) and Ecole Polytechnique Fédérale de Lausanne (Switzerland) report that cells destined to become nerve cells in developing mouse embryos use two different signals spreading from opposite sides of the spinal cord – the back and belly side – to measure their position accurately. Based on this map, they turn into the appropriate nerve cell type. The research was funded by the European Research Council and Wellcome.
The team of biologists, physicists and engineers found that the amounts of the two signals originating from the back and belly sides of the body affect gene activity in developing nerve cells. Based on this gene activity in early development, the cells turn into the appropriate nerve cell type for that position in the spinal cord.
"We've made an important step in understanding how the diverse cell types in the spinal cord of a developing embryo are organised in a precise spatial pattern. The quantitative measurements and new experimental techniques we used, as well as the combined effort of biologists, physicists and engineers were key. This allowed us to gain new insight into the exquisite accuracy of embryonic development and revealed that cells have remarkable ability of to orchestrate precise tissue development," says Anna Kicheva, Group Leader at IST Austria.
"We have shed light on the long-standing question of how developing tissues produce the right cells in the right place in the right numbers," says James Briscoe, Group Leader at the Francis Crick Institute. "It's likely that similar strategies are used in other developing tissues and our findings might be relevant to these cases. In the long run this will help inform the use of stem cells in approaches such as tissue engineering and regenerative medicine. However, there is still much more to learn and we need to continue developing these interdisciplinary collaborations to further our biological understanding."
The paper 'Decoding of position in the developing neural tube from antiparallel morphogen gradients' is published in Science.
###
Media Contact
Greta Keenan
[email protected]
020-379-65252
@thecrick
http://www.crick.ac.uk
############
Story Source: Materials provided by Scienmag