Credit: Illustration by Kuo-Fu Tseng, courtesy of Oregon State University.
CORVALLIS, Ore. – A study published today offers a new understanding of the complex cellular machinery that animal and fungi cells use to ensure normal cell division, and scientists say it could one day lead to new treatment approaches for certain types of cancers.
The research revealed a totally unexpected behavior about a "motor" protein that functions as chromosomes are segregated during cell division. The findings were published in Nature Communications.
The work was led by Weihong Qiu, an assistant professor of physics in the College of Science at Oregon State University, in collaboration with researchers from Henan University in China and the Uniformed Services University of the Health Sciences in Maryland.
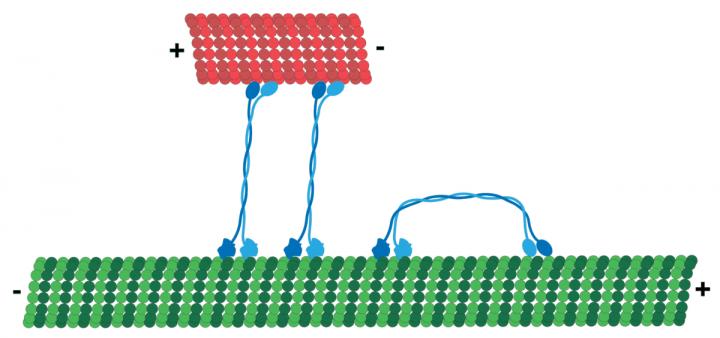
Motor proteins are tiny molecular machines that convert chemical energy into mechanical work. They are the miniature "vehicles" of a cell, and move on a network of tracks commonly referred to as the cytoskeleton. They shuttle cellular cargos between locations and generate forces to position chromosomes. But in spite of intensive research efforts over many years, mechanisms underlying the actions of many motor proteins are still unclear.
In this study, researchers focused on a particular motor protein, called KlpA, and used a high-sensitivity light microscopy method to directly follow the movement of individual KlpA molecules on the cytoskeleton track. They discovered that KlpA is able to move in opposite directions – an unusual finding. KlpA-like motor proteins are thought to be exclusively one-way vehicles.
The researchers also discovered that KlpA contains a gear-like component that enables it to switch direction of movement. This allows it to localize to different regions inside the cell so it can help ensure that chromosomes are properly divided for normal cell division.
"In the past, KlpA-like motor proteins were thought to be largely redundant, and as a result they haven't been studied very much," Qiu said.
"It's becoming clear that KlpA-like motors in humans are crucial to cancer cell proliferation and survival. Our results help better understand other KlpA-like motor proteins including the ones from humans, which could eventually lead to novel approaches to cancer treatment."
Qiu and colleagues say they are excited about their future research, which may uncover the design principle at the atomic level that allows KlpA to move in opposite directions. And there may be other applications.
"KlpA is a fascinating motor protein because it is the first of its kind to demonstrate bidirectional movement," Qiu said. "It provides a golden opportunity for us to learn from Mother Nature the rules that we can use to design motor protein-based transport devices. Hopefully in the near future, we could engineer motor protein-based robotics for drug delivery in a more precise and controllable manner."
###
The work was done with partial support from the National Science Foundation.
Media Contact
Weihong Qiu
[email protected]
541-737-7377
@oregonstatenews
http://www.orst.edu
############
Story Source: Materials provided by Scienmag





