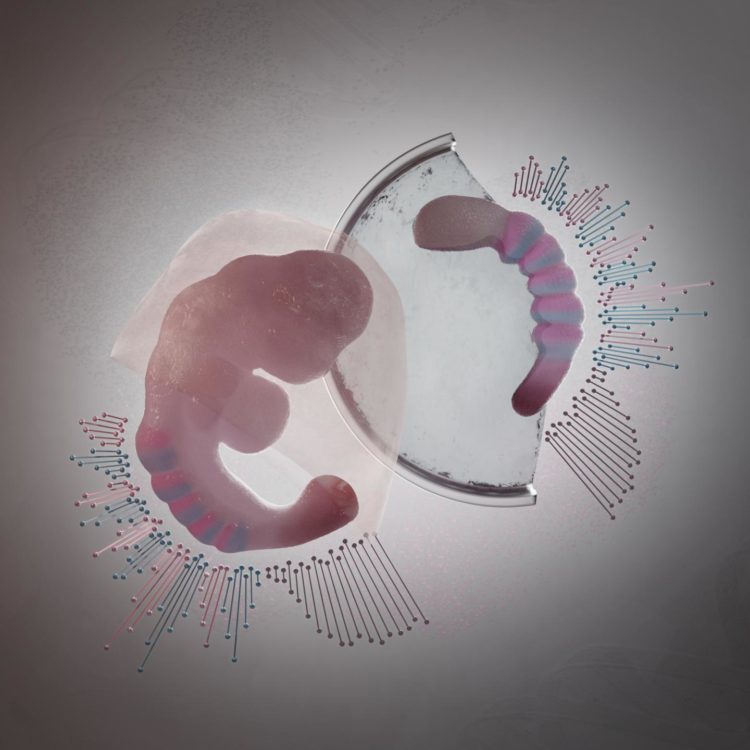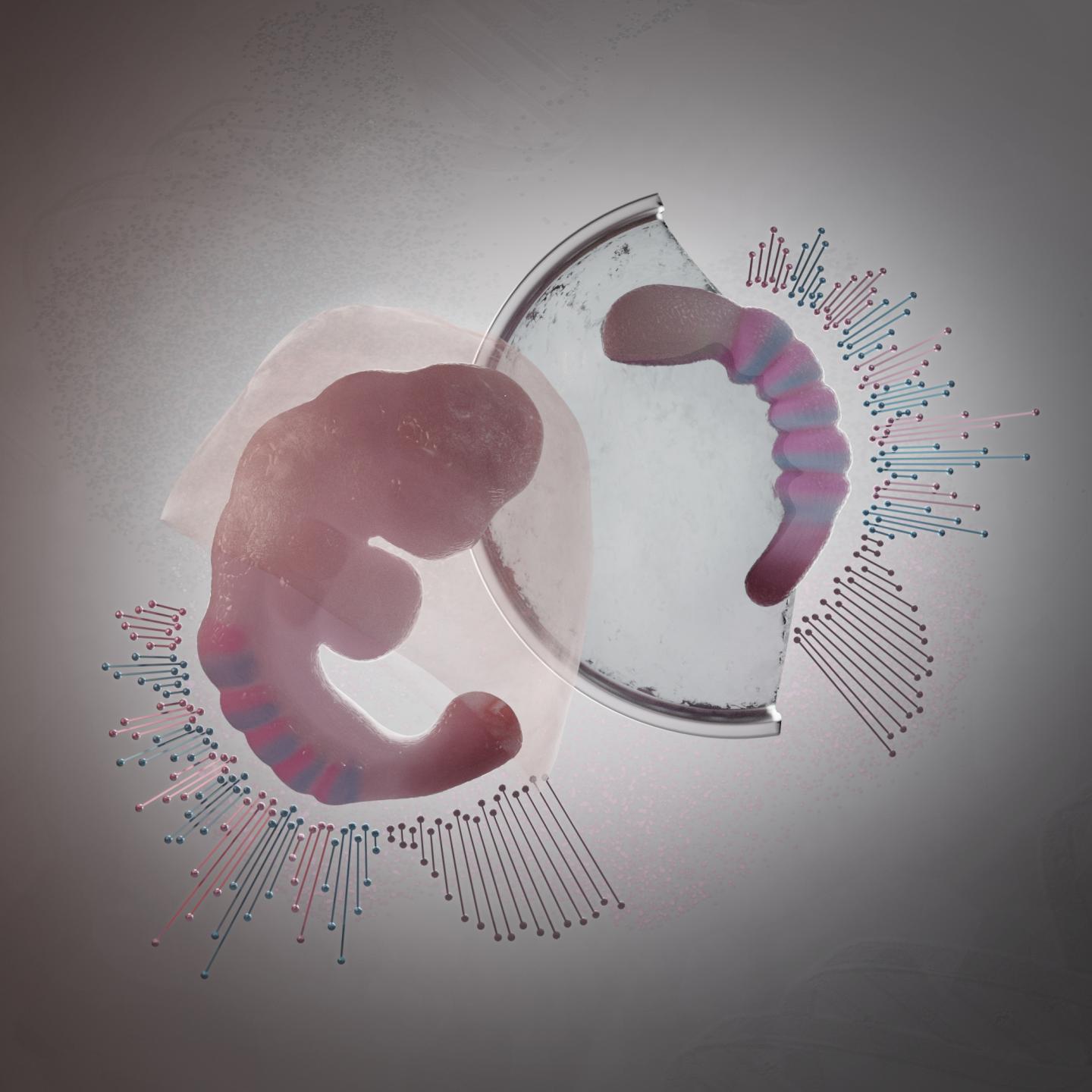
Credit: Núria Taberner, ©Hubrecht Institute
Scientists from the Hubrecht Institute (KNAW) and the University of Cambridge (UK) have managed to generate complex embryo-like structures from mouse embryonic stem cells. These structures, called gastruloids, can now for the first time grow somites, the blocks of tissue that later develop into the vertebrae and muscles of the embryo. It is the first time that scientists managed to generate such advanced embryo-like structures that represent this stage of embryonic development, which occurs after implantation in the uterus. This model system allows these later stages of embryonic development to be studied in a dish. The results of their study were published in Nature on the 19th of February.
During embryogenesis, a fertilized egg develops into a complete organism. However, much is still unknown about the processes that direct embryonic development in mammals. For example, how do mammals regulate the number of vertebrae that make up their spines, or on which side of the body the heart should develop? Every now and then, something goes wrong in these processes. How does this happen? And can we prevent this from happening? Which compounds are beneficial for embryonic development, and which ones are bad? Studies that address such questions often require the use of mouse embryos. These embryos however develop within the uterus and it is difficult to obtain them in large numbers. Scientists from the laboratories of Alexander van Oudenaarden and Katharina Sonnen (Hubrecht Institute) and the laboratory of Alfonso Martinez Arias (University of Cambridge) are therefore developing an alternative: gastruloids, embryo-like structures generated from stem cells in the lab.
Somites
In 2014, these scientists for the first time grew embryo-like structures, called gastruloids, from mouse stem cells. Now, they have further improved the culture conditions to increase the similarities between gastruloids and embryos, which has resulted in gastruloids that grow somites. Somites are small “blocks” of tissue that line the back of the embryo, and will later form the vertebrae and skeletal muscles. The current gastruloids are thus more complex and resemble mouse embryos in more detail than previous models. Susanne van den Brink (Hubrecht Institute): “This is the first time we have managed to generate such complex embryo-like structures that recapitulate the embryonic stages of development that normally happen after implantation in the uterus.” Vincent van Batenburg (Hubrecht Institute) adds: “It is fascinating to see that a homogenous group of cells can organize itself into an embryo-like structure that generates somites in a dish.”
Gastruloids and embryos
The scientists made a detailed comparison between gastruloids and embryos by investigating what cell types and tissues are present in these structures. In addition, they investigated where these cell types and tissues are located along the head-to-tail body axis, in both gastruloids and embryos. As the combination of active genes determines the cell type, they could investigate these cell types by measuring which genes are active in which location, in both embryos and gastruloids.
State-of-the-art
To this end, the scientists used state-of-the art technologies, such as single-cell sequencing and tomo-sequencing. Single-cell sequencing can be used to measure the total set of active genes in each individual cell, but it does not provide information about the location of these cells. In contrast, tomo-sequencing is a method in which the embryo or gastruloid is sectioned into thin slices, in this case from head to tail, after which the activity of genes is measured in each of these sections.
A suitable model system
The combination of single-cell sequencing and tomo-sequencing enabled the scientists to measure exactly which cell types are present in gastruloids, and where these cell types were positioned along the head-to-tail axis of gastruloids and embryos. Susanne van den Brink (Hubrecht Institute): “We discovered that gastruloids are very similar to embryos, which makes them a very suitable model system to study embryonic development.” Anna Alemany: “An important difference between embryos and gastruloids however, is that gastruloids do not have a brain or placenta. Therefore, these structures are not viable.”
Studying developmental processes
With this study, the scientists show that gastruloids can now be used to study more complex processes that happen in embryos, such as the processes that regulate somite formation. Alexander van Oudenaarden: “A main advantage of this model system is that gastruloids can be grown in large numbers. This means that we may be able to use them to test new drugs for developmental defects, or to test which compounds are toxic for developing embryos.” Scientists can also use gastruloids to study how embryonic defects appear, such as defects during the formation of the heart and muscles.
###
Publication
Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Susanne C. van den Brink*, Anna Alemany*, Vincent van Batenburg*, Naomi Moris, Marloes Blotenburg, Judith Vivié, Peter Baillie-Johnson, Jennifer Nichols, Katharina F. Sonnen,Alfonso Martinez Arias & Alexander van Oudenaarden. Nature, 2020.
* Shared first authors
Interactive website: https:/
Alexander van Oudenaarden is director of the Hubrecht Institute, group leader, professor of Quantitative Biology of Gene Regulation at the UMC Utrecht and Utrecht University and Oncode Investigator.
Katharina Sonnen is group leader at the Hubrecht Institute.
Alfonso Martinez Arias is group leader and professor of Developmental Mechanics at the Department of Genetics in the Cambridge University.
About the Hubrecht Institute
The Hubrecht Institute is a research institute focused on developmental and stem cell biology. It encompasses 23 research groups that perform fundamental and multidisciplinary research, both in healthy systems and disease models. The Hubrecht Institute is a research institute of the Royal Netherlands Academy of Arts and Sciences (KNAW), situated on Utrecht Science Park. Since 2008, the institute is affiliated with the UMC Utrecht, advancing the translation of research to the clinic. The Hubrecht Institute has a partnership with the European Molecular Biology Laboratory (EMBL). For more information, visit https:/
About the Cambridge of University
The mission of the University of Cambridge is to contribute to society through the pursuit of education, learning and research at the highest international levels of excellence. To date, 109 affiliates of the University have won the Nobel Prize. Founded in 1209, the University comprises 31 autonomous Colleges, which admit undergraduates and provide small-group tuition, and 150 departments, faculties and institutions. Cambridge is a global university. Its 19,000 student body includes 3,700 international students from 120 countries. Cambridge researchers collaborate with colleagues worldwide, and the University has established larger-scale partnerships in Asia, Africa and America. The University sits at the heart of the ‘Cambridge cluster’, which employs 60,000 people and has in excess of £12 billion in turnover generated annually by the 4,700 knowledge-intensive firms in and around the city. The city publishes 341 patents per 100,000 residents. http://www.
Funding for this study
This work was supported by an European Research Council Advanced grant (ERC-AdG 742225-IntScOmics), a Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) TOP award (NWO-CW 714.016.001) and the Foundation for Fundamental Research on Matter, financially supported by NWO (FOM-14NOISE01) to S.C.v.d.B., A.A., V.v.B., M.B., J.V. and A.v.O., a Biotechnology and Biological Sciences Research Council (No. BB/ P003184/1), Newton Trust (INT16.24b) and Medical Research Council (MR/R017190/1) grant to A.M.A., a Newnham College Cambridge Junior Research Fellowship to N.M. and a studentship from the Engineering and Physical Sciences Research Council to P.B.-J. The Cambridge Stem Cell Institute is supported by core funding from the Wellcome Trust and Medical Research Council; J.N. was funded by the University of Cambridge and K.F.S. by core funding from the Hubrecht Institute. This work is part of the Oncode Institute, which is partly financed by the Dutch Cancer Society.
Media Contact
Melanie Fremery
[email protected]
31-683-596-548
Related Journal Article
http://dx.