DENVER, April 22, 2022 – A randomized controlled trial evaluates the safety of furosemide in preterm infants at risk of bronchopulmonary dysplasia. Findings from the study will be presented during the Pediatric Academic Societies (PAS) 2022 Meeting, taking place April 21-25 in Denver.
Credit: Duke Clinical Research Institute
DENVER, April 22, 2022 – A randomized controlled trial evaluates the safety of furosemide in preterm infants at risk of bronchopulmonary dysplasia. Findings from the study will be presented during the Pediatric Academic Societies (PAS) 2022 Meeting, taking place April 21-25 in Denver.
The objective of the study was to evaluate the safety and preliminary efficacy of furosemide in preterm infants at risk of developing bronchopulmonary dysplasia.
Researchers found that in preterm infants at high risk for bronchopulmonary dysplasia, adverse events occurred in nearly all infants regardless of treatment group. Furosemide increased the risk of electrolyte adverse events. There was no difference in hearing loss, nephrocalcinosis, or bronchopulmonary dysplasia/death.
“The drug furosemide is commonly used in premature infants hospitalized in the neonatal intensive care unit to prevent bronchopulmonary dysplasia, a type of chronic lung disease,” said Rachel G. Greenberg, MD, MB, MHS, associate professor of pediatrics with the division of neonatal-perinatal medicine at Duke University Medical Center, and program director with Duke Neonatal-Perinatal Medicine Fellowship. “Unfortunately, very little data is available to help neonatologists understand whether furosemide is safe and effective. Our study, which was performed at 17 centers within the NICHD Pediatric Trials Network, was the first randomized controlled trial to evaluate the safety and preliminary efficacy of furosemide in premature infants at risk for developing bronchopulmonary dysplasia.”
Dr. Greenberg added: “We found that infants exposed to furosemide had more electrolyte problems but no greater risk of overall safety events, including kidney stones and hearing problems. The results of this study will help the practicing neonatal provider and help to design future trials.”
Dr. Greenberg will present “Safety of Furosemide in Preterm Infants at Risk of Bronchopulmonary Dysplasia: A Randomized Controlled Trial” on Saturday, April 23 at 1:15 p.m. MDT. Reporters interested in an interview with Dr. Greenberg should contact [email protected].
The PAS Meeting connects thousands of pediatricians and other health care providers worldwide. For more information about the PAS Meeting, please visit www.pas-meeting.org.
###
About the Pediatric Academic Societies Meeting
The Pediatric Academic Societies (PAS) Meeting is the premier North American scholarly child health meeting. The PAS Meeting connects thousands of pediatricians and other health care providers worldwide. The PAS Meeting is produced through a partnership of four pediatric organizations that are leaders in the advancement of pediatric research and child advocacy: American Pediatric Society, Society for Pediatric Research, Academic Pediatric Association and American Academy of Pediatrics. For more information, please visit www.pas-meeting.org. Follow us on Twitter @PASMeeting, Instagram PASMeeting and #PAS2022, and like us on Facebook PASMeeting.
—
Abstract: Safety of Furosemide in Preterm Infants at Risk of Bronchopulmonary Dysplasia: A Randomized Controlled Trial
Topic
Neonatal Clinical Trials
Presenting Author
Rachel G. Greenberg, MD, MB, MHS
Organization
Duke Clinical Research Institute
Background
Furosemide is commonly used in preterm infants to prevent bronchopulmonary dysplasia (BPD) without evidence that furosemide is safe or reduces the incidence of moderate/severe BPD.
Objective
To evaluate the safety and preliminary efficacy of furosemide in preterm infants at risk of developing BPD.
Design/Methods
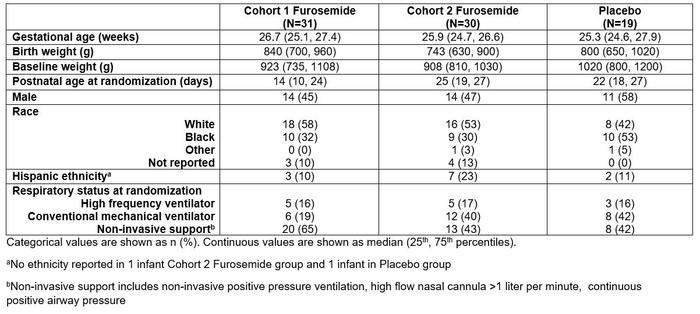
This multi-center, randomized, dose-escalating, placebo-controlled trial (NCT02527798; IND 120,933) included infants < 29 weeks gestational age (GA) and 7-28 days postnatal age receiving positive airway pressure or mechanical ventilation. Infants were randomized 3:1 (furosemide:placebo) into 2 cohorts (n=40 per cohort) with escalating doses of furosemide to a maximum of 1 mg/kg IV (or enteral equivalent) every 24 hours (Cohort 1) or 1 mg/kg IV (or enteral equivalent) every 6 hours (Cohort 2) for 28 days. The primary outcome was safety as determined by the incidence of any adverse events (AE). Multivariable logistic regression with adjustment for GA was used to estimate the treatment effect on other safety and efficacy endpoints including the composite outcome of moderate-severe BPD (by NIH consensus definition) or death, hearing loss (assessed via site preference), serum electrolyte AEs, and nephrocalcinosis.
Results
Of 82 infants randomized, 80 received study drug and were included in analyses (Table 1). Median total duration of study drug was 28 days (range: 2-29) in the furosemide group and 28 days (range: 5-29) in the placebo group. A total of 293 AEs were reported in 74/80 (93%) infants, including 223 AEs among the 56 (92%) infants who received furosemide and 70 AEs among the 18/19 (95%) infants who received placebo (p=0.94, Table 2). The most common serum electrolyte AEs were high bicarbonate levels (73%), hypochloremia (39%), and hyperchloremia (33%). The odds ratio for having a serum electrolyte AE was 4.46 (95% CI: 1.06- 21.7; P=0.048) for Cohort1/Furosemide vs. placebo and 7.89 (95% CI: 1.50-61.91; P=0.023) for Cohort 2/Furosemide vs. placebo. BPD or death occurred in 39/53 (74%) of infants who received furosemide and 12/18 (67%) of infants who received placebo (Table 3). After adjustment for GA, there was no difference between groups in the odds of having BPD or death (P=0.32), hearing loss (P=0.78), or nephrocalcinosis (P=0.39).
Conclusion(s)
In preterm infants at high risk for BPD, adverse events occurred in nearly all infants regardless of treatment group. Furosemide increased the risk of electrolyte AEs. There was no difference in hearing loss, nephrocalcinosis, or BPD/death.
Tables and Images
Table 1. Demographics and clinical characteristics.
PAS abstract_Table1.JPG
Table 2. Adverse events.
PAS abstract_Table2.JPG
Table 3. Secondary end points.
PAS abstract_Table3.JPG
Method of Research
Randomized controlled/clinical trial




