Credit: Department of Chemistry, HKUST
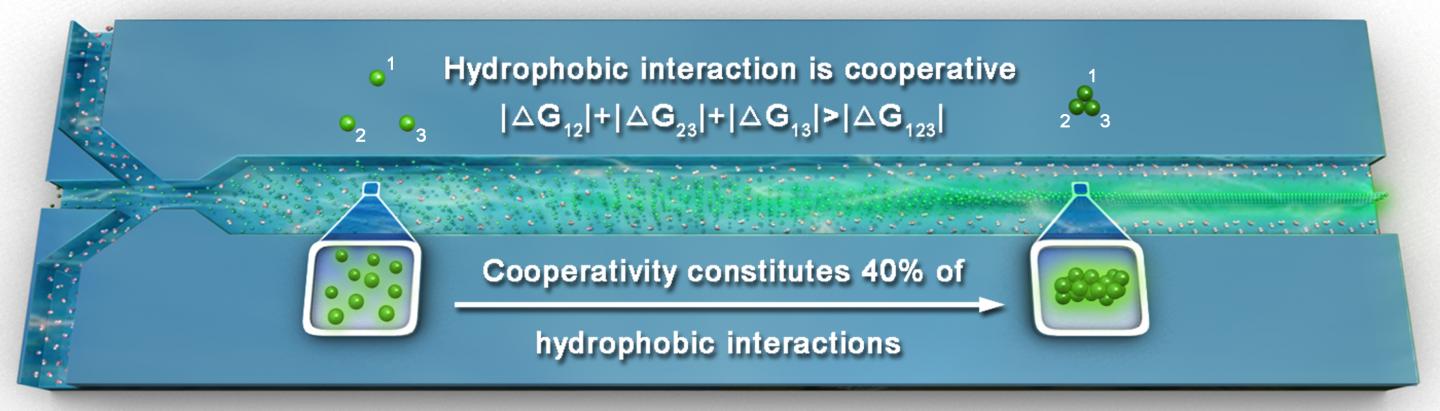
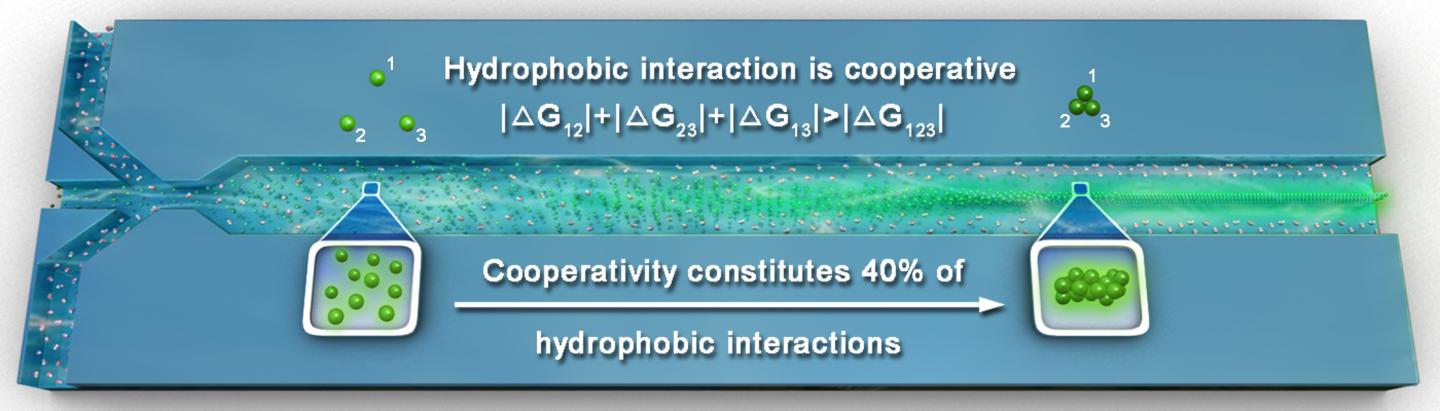
Hydrophobic interactions is one major type of intermolecular force that plays a vital role in many life processes in Chemistry and Physics. In biological systems, hydrophobic interactions can stabilize the internal cores of proteins and form lipid vesicles that store nutrients in our cells.In proteins, hydrophobic interactions can stabilize the internal cores and form lipid vesicles that store nutrients in our cells. What is so intriguing about hydrophobic interaction is that it exhibits a cooperative property called cooperativity, which does not exist in other fundamental intermolecular forces, such as dipole-dipole interactions and Van der Waals forces. Cooperativity means that in the presence of multiple molecules (at least more than two), the overall strength of the interaction between the molecules is much greater than that when there are only two molecules acting in pairs.
A major gap in the textbook knowledge of hydrophobic interaction and its cooperativity that have profound implications in many fundamental processes in nature is: to what extent cooperativity contributes to hydrophobic interactions that stabilize the assembly of macromolecules? One gigantic roadblock to solving this long-standing puzzle is the extreme difficulty to quantify cooperativity by experiments, as cooperativity is originated from the collective motions of water hydrogen bond networks surrounding hydrophobic solutes.
In a breakthrough, scientists from The Hong Kong University of Science and Technology overcame these challenges by designing an innovative microfluidic mixer that monitors the fluorescence induced by hydrophobic aggregation. This scientific advance not only allows the quantification of molecular hydrophobic interaction and its cooperativity in bulk solution, but also provides a clear and quantitative evidence for the critical role of cooperativity in hydrophobic aggregation that is being consolidated by their kinetic nucleation-growth theory.
Their findings were published in the journal Nature Communications on May 31, 2017 (doi: 10.1038/ncomms15639).
"To quantify hydrophobic interactions, we real-time monitored hydrophobic aggregation in bulk solution at microsecond time scale," said Prof. Xuhui Huang, corresponding author of the manuscript. "To achieve this, we probed fluorescence induced by aggregation upon rapid mixing of water and hydrophobic solute using the microfluidic device. We then fitted the measured fluorescence to the kinetics nucleation-growth theory".
"The state-of-the-art microfluidic device allows us to track the aggregation of the solute molecule at very short, microsecond timescales due to the fine dimensions of the solute jet (at submicron in diameter) focused by microfluidic flow. Our results demonstrated that the attachment of a hydrophobic monomer to its aggregate in water occurs at sub-microsecond." Prof. Shuhuai Yao, the other corresponding author further elaborated.
###
Media Contact
Johnny Tam
[email protected]
852-235-88556
http://www.ust.hk
Related Journal Article
http://dx.doi.org/10.1038/ncomms15639
############
Story Source: Materials provided by Scienmag