In the ever-evolving battle against cancer, researchers from the Japan Advanced Institute of Science and Technology (JAIST) are making remarkable strides by combining advanced nanotechnology and innovative therapeutic methods. Led by Professor Eijiro Miyako, this research team has developed multifunctional nanoparticles that leverage magnetic ionic liquids for targeted cancer treatment. Their findings, published in the journal Small Science on March 3, 2025, highlight a new frontier in cancer theranostics, making it possible to direct treatment with unprecedented precision.
Traditional cancer therapies such as chemotherapy, radiation, and surgery have long been the cornerstones of oncological care. However, these methods can indiscriminately harm healthy tissues, leading to a range of debilitating side effects. The urgent need for more refined and effective treatment options has catalyzed research into targeted therapies—therapies specifically designed to distinguish between malignant and healthy cells. This quest for precision medicine has led to groundbreaking innovations in how we approach cancer treatment.
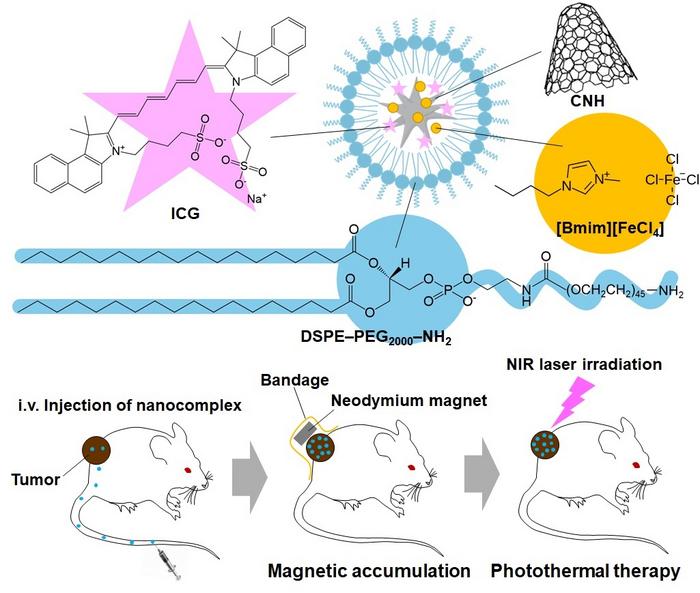
At the core of this innovation are nanoparticles, microscopic carriers designed to deliver therapeutic agents directly to tumors. The research team aims not only to target the cancer cells but also to enhance the efficacy of treatment through the incorporation of photothermal therapy. This method employs nanoparticles that absorb specific wavelengths of light and convert them into heat to destroy cancer cells selectively. When these nanoparticles are illuminated with near-infrared (NIR) laser light, they generate sufficient heat to induce apoptosis in nearby tumor cells.
The primary challenge with utilizing nanoparticles in a clinical setting has been ensuring their accumulation at tumor sites, an obstacle that the research team has tackled head-on. By modifying carbon nanohorns—spherical graphene-based nanostructures utilized for drug delivery—with magnetic ionic liquids, the team created a new class of nanoparticles capable of being guided magnetically to tumor targets. This innovative approach not only enhances dispersion within the body but also leverages the inherent magnetic properties of the liquid to facilitate targeted delivery.
To make the nanoparticles biocompatible and water-soluble, the team employed a polyethylene glycol (PEG) coating, addressing the hydrophobic nature of both the carbon nanohorns and the ionic liquid they modified. This step is vital in ensuring that the nanoparticles remain stable and effective in biological environments, dramatically increasing their potential suitability for in vivo applications. Furthermore, by integrating indocyanine green—a fluorescent dye—the researchers incorporated a mechanism for real-time tracking of the nanoparticles, allowing for enhanced monitoring throughout the therapeutic process.
In their experiments, the team conducted rigorous tests to evaluate the effectiveness of these nanoparticles against cancer cells derived from mouse colon carcinoma (Colon26). The results were striking: the nanoparticles exhibited a photothermal conversion efficiency of 63%, enabling them to induce significant cytotoxic effects after exposure to an 808 nm NIR laser. Administered in vivo to mice with induced tumors, the magnetically guided nanoparticles successfully concentrated at tumor sites, raising the temperature to levels sufficient for tumor ablation.
After six treatment sessions using this novel approach, the treated mice showed complete tumor elimination, a testament to the nanoparticles’ effectiveness when combined with magnetic guidance and photothermal therapy. This contrasts sharply with control groups where nanoparticles were not magnetically targeted; those tumors displayed rapid regrowth, highlighting the crucial role of precise targeting in achieving therapeutic success.
Professor Miyako articulates the significance of this research, emphasizing how the incorporation of multiple modalities—thermal destruction, magnetic targeting, and chemotherapeutic effects—provides a multifaceted approach to combating cancer. This strategy could revolutionize cancer treatment by merging techniques that traditionally function in isolation into an integrated, holistic model, thereby increasing the overall effectiveness of therapies while minimizing damage to surrounding healthy tissue.
Despite these promising results, further research is imperative. The study calls for additional safety testing to ascertain the long-term implications of using these nanoparticles within living organisms. Additionally, the development of sophisticated endoscopic laser systems would be necessary to treat deeper-seated tumors, unlocking the potential of this groundbreaking technique for a broader range of patients in various stages of cancer.
The implications of this work extend well beyond just treating tumors. It opens doors to novel research opportunities into how we can manipulate nanomaterials for various therapeutic applications. By harnessing the synergies offered by nanotechnology and learning more about the biological behavior of these nanoparticles, we can pave the way for new delivery mechanisms for a range of drugs, potentially leading to advances in treating other chronic and complex diseases.
In summary, the research led by Professor Miyako marks a significant advancement in the domain of cancer treatment, combining principles of nanotechnology with targeted therapeutic strategies. This innovative approach transforms the landscape of cancer treatment by offering hope for better outcomes through enhanced precision and effectiveness compared to traditional methods. As research continues in this exciting area, we may soon witness a new era in personalized medicine where each patient’s cancer can be treated with tailored approaches designed to optimize therapeutic outcomes.
The future looks promising for the integration of magnetic ionic liquids in cancer theranostics, potentially changing the way we think about and manage cancer at a fundamental level.
Subject of Research: Targeted Cancer Therapy
Article Title: Multifunctional Magnetic Ionic Liquid-Carbon Nanohorn Complexes for Targeted Cancer Theranostics
News Publication Date: 3-Mar-2025
Web References: https://onlinelibrary.wiley.com/doi/full/10.1002/smsc.202400640
References: 10.1002/smsc.202400640
Image Credits: Eijiro Miyako from JAIST
Keywords: Cancer, Nanoparticles, Photothermal Therapy, Targeted Therapy, Nanotechnology, Magnetic Ionic Liquids, Therapeutics
Tags: advanced oncology methodsenhancing efficacy of cancer therapiesheat-activated nanotechnologyinnovative cancer theranosticsJapan Advanced Institute of Science and Technologymagnet-guided nanoparticlesmultifunctional nanoparticlesphotothermal therapy in cancerprecision cancer therapyProfessor Eijiro Miyako researchreducing cancer treatment side effectstargeted cancer treatment